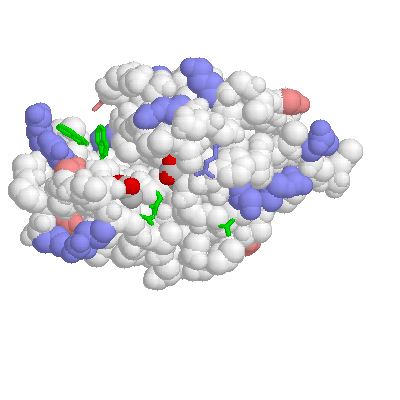
CARBOXYPEPTIDASE A WITHOUT SUBSTRATE:
Notice tyr 248 pointed up and away from the active site.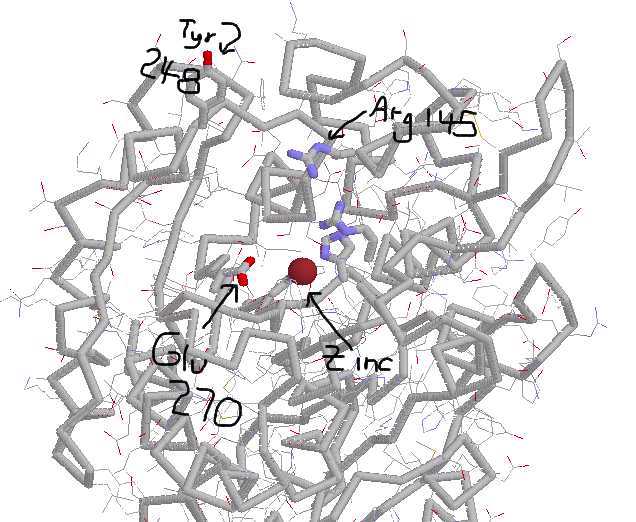
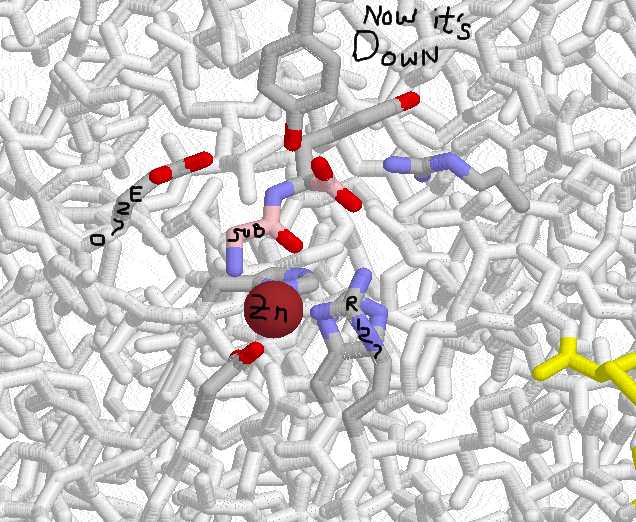
CARBOXYPEPTIDASE A PLUS SUBSTRATE:
Now the tyr has swung down into the binding pocket.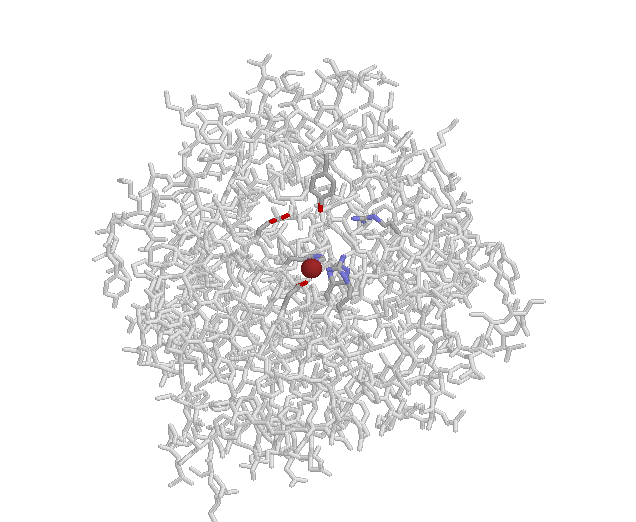
SERINE PROTEASES
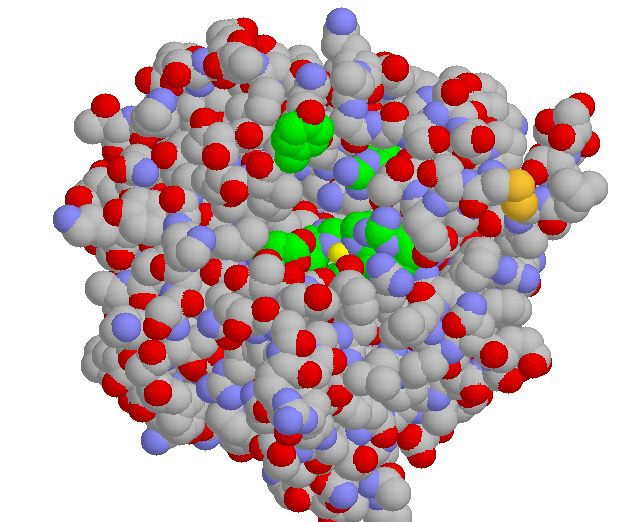
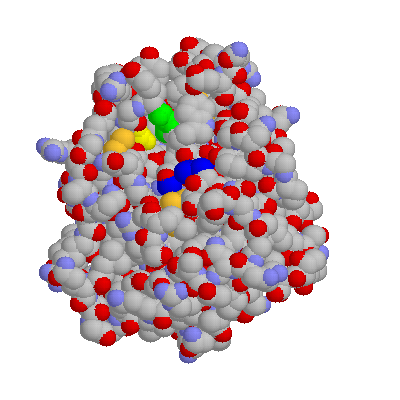
Here's trypsin. The binding pocket is in yellow (asp) and green (gly). The carbons on the ser-his-asp at the active site have been colored blue. Those "gold" items are cystines.
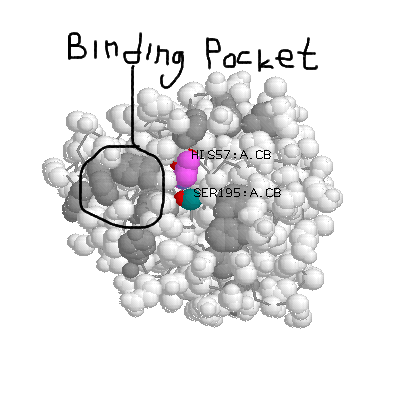
Here's chymotrypsin with its binding pocket dominated by (grey) aromatics.
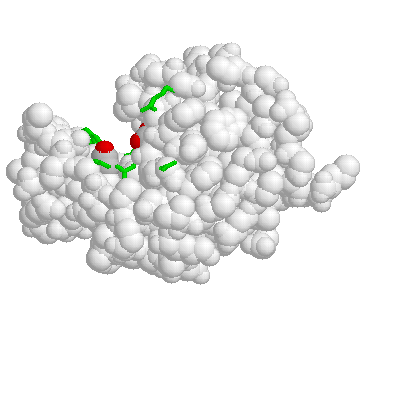
LYSOZYME
The binding groove or cleft is evident here. Asp 52 is on the left and glu 35 is on the right--both are grey with red oxygens.
Now we've moved above the groove. Asp 52 is on the left and glu 35 is on the right--both are grey with red oxygens. The lowest green stick figure is arg 114. The rings are the tryptophans (62 and 63). Asp 101 is the highest-placed green stick figure.
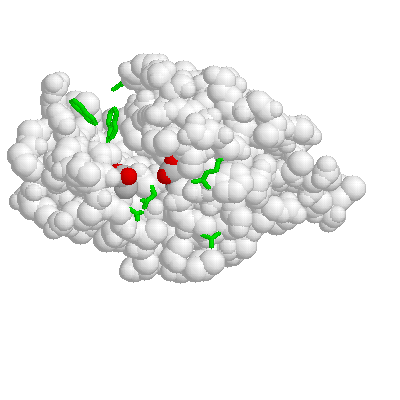
Here's one more with acidic sidechains in pink and basic ones in blue. No real point except that the charged groups are on the surface. Somehow arg 114 escaped being changed to blue.