|
 |

 |
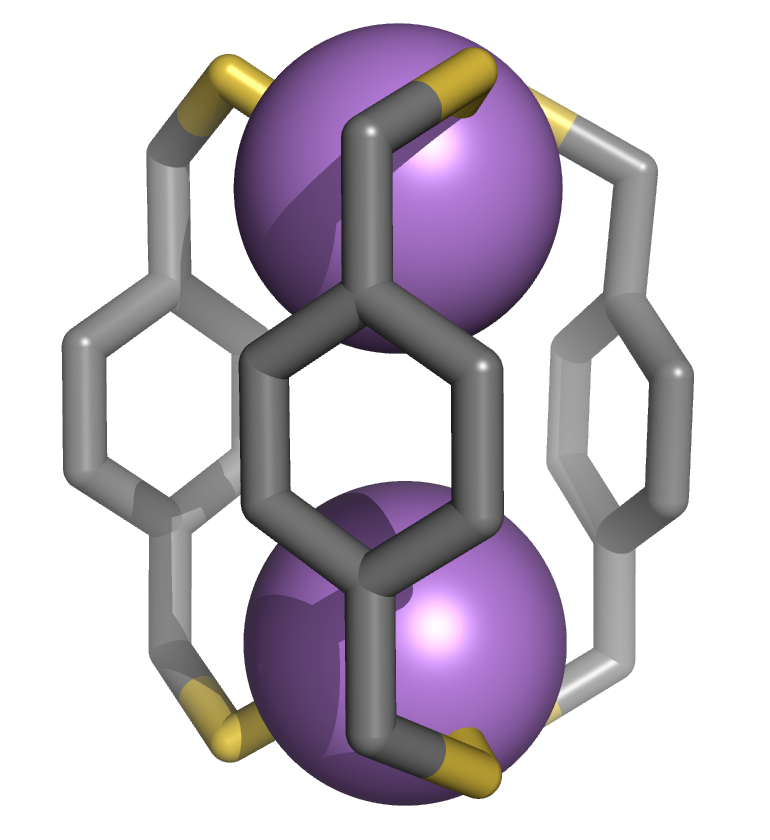
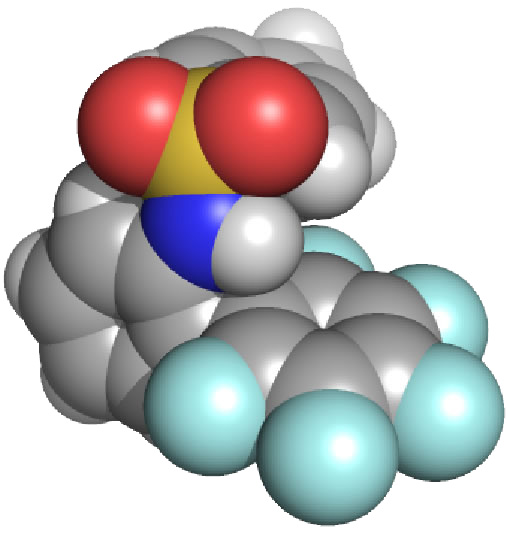
| We are elaborating a design strategy for forming coordination capsules comprised of toxic metal ions or main group elements (such as arsenic or lead). We have recently shown that ligand H2L binds arsenic(III) within a very stable As2L3 cage. Surprisingly, two As2L2Cl2 macrocycles were also isolated as intermediates in the reaction. Applications in metal remediation, both environmental and medical, are envisioned by designing the ligands to target a specific toxic or hazardous metal ion. Furthermore, incorporating these nanoscale coordination capsules into extended solid-state structures leads to applications in materials science, and the novel cavity environments in these capsules will lead to unusual host-guest properties. |
| |
 |
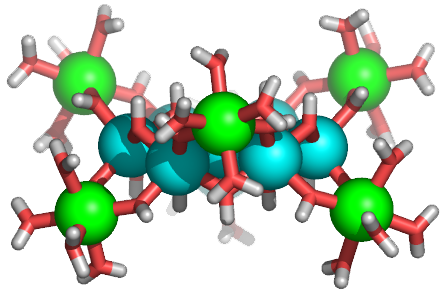
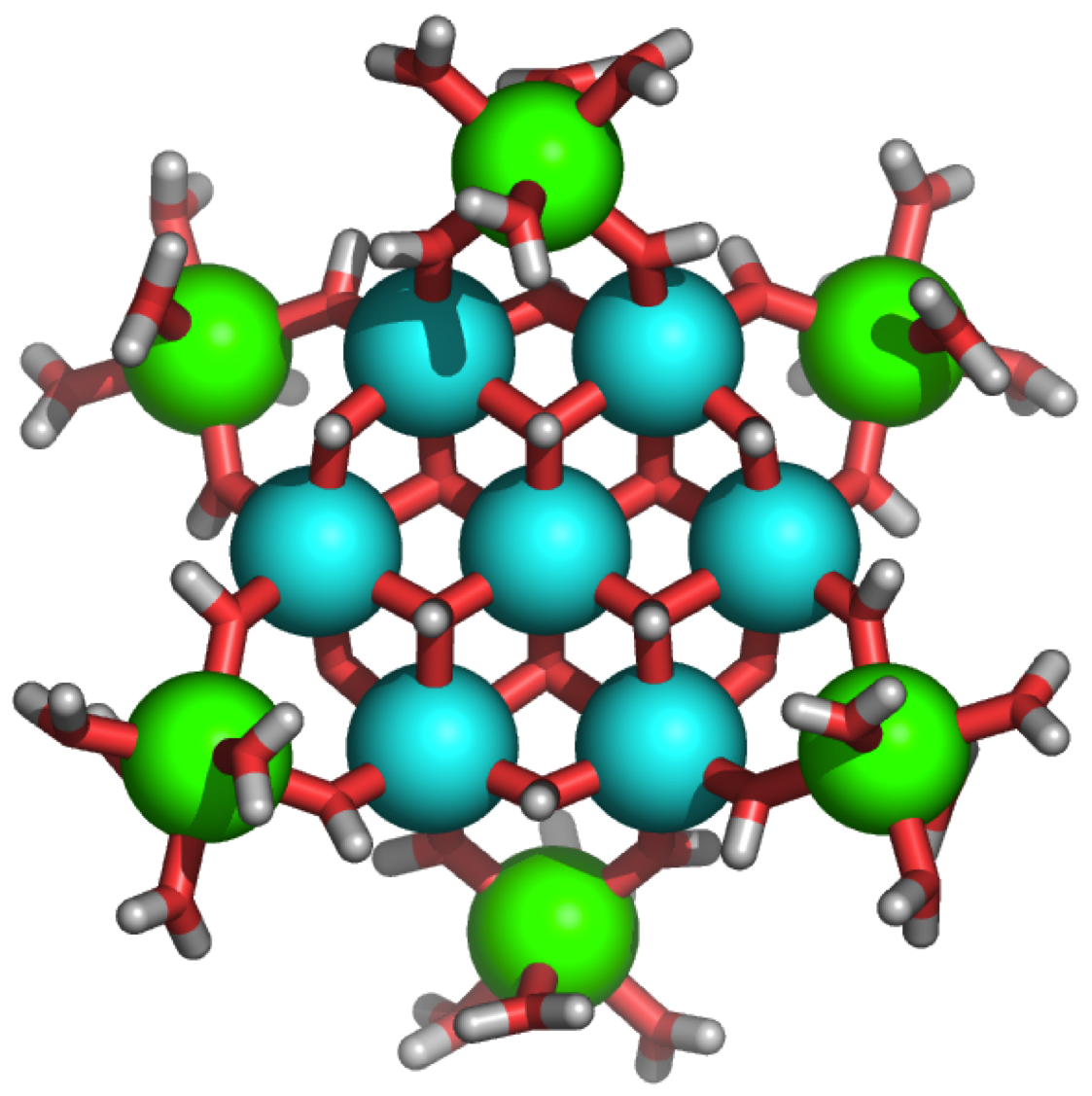
| Developing predictive design strategies to prepare inorganic cluster compounds has attracted much research interest, due in part to the potential applications of these novel materials. We have developed an unusual new synthetic strategy for preparing discrete inorganic clusters, and we have used this strategy to prepare the first crystalline example of an inorganic tridecameric Ga cluster (Ga13, see figure). This same facile synthetic methodology also yields the analogous tridecameric aluminum complex and a mixed Ga/In tridecameric cluster in preparative scales after several days. We are currently exploring the use of these inorganic aggregates as synthons for the generation of a wider range of particles and assemblies via exchange of the peripheral water ligands with appropriate organic ligands. |
| |
 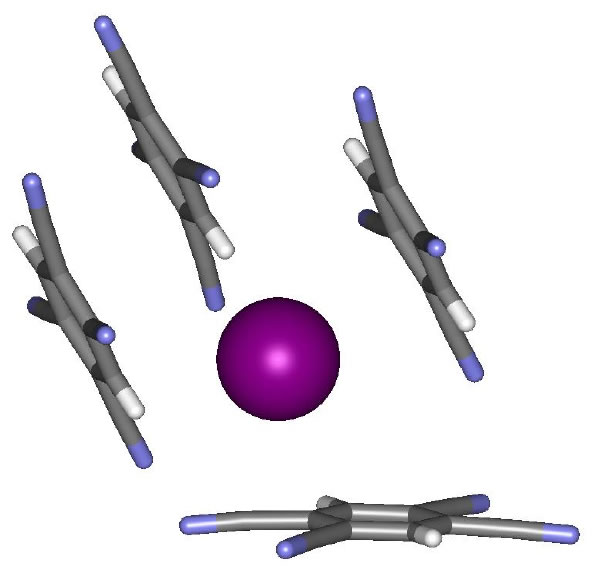 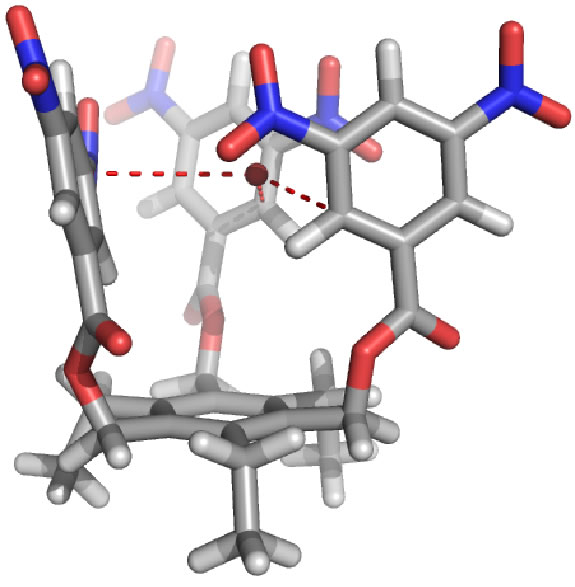 |
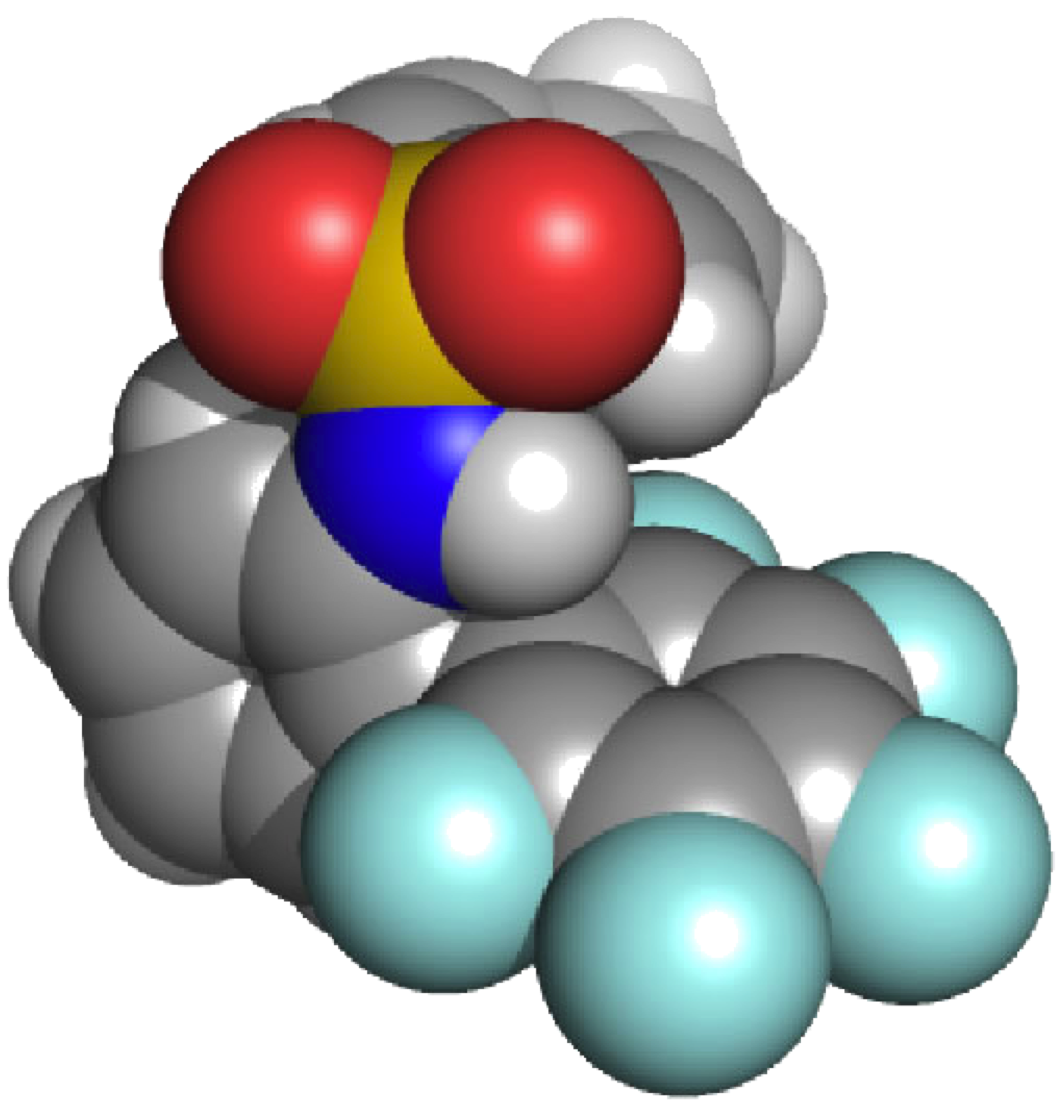
| One area of research interest in the Darren W. Johnson laboratory is the study of how anions interact with electron-deficient aromatic rings. We have shown in a model system containing both a hydrogen bond donor and a pentafluorophenyl substituent that the presence of the electron-deficient aromatic ring enhances halide binding in solution. We have also highlighted in the solid state and in silico our observations that anions can form as many as four different attractive interactions with electron-deficient aromatic rings. Recently we have utilized this design strategy to develop tripodal anion receptors that utilize only electron-deficient aromatic rings to bind anions. We strive to understand this interaction in greater detail by improving receptor design and developing new electron-deficient arenes to bind anions. The anion binding selectivity of electron-deficient aromatic rings will be investigated in due course. |
|
 |
| |