 The image shown on the left was taken with a high resolution Transmission
Electron Microscope. The image represents a projection through a very thin
(ca. 200Angstroms) slice of the mineral cordierite (Mg2Al4Si5O18).
The dark areas represent hollow channels through the structure while the
white spots can be equated with regions of high atomic density. The distance
betwen the dark "spots" represents approximately 9.6 Angstroms! This figure
represents conclusive evidence for the "ordered atomic arrangement" characteristic
of minerals. Note the periodicity and the symmetry. The structure in two
dimensions, consists of an interlocking network of hexagonal (6-fold) rings.
The image shown on the left was taken with a high resolution Transmission
Electron Microscope. The image represents a projection through a very thin
(ca. 200Angstroms) slice of the mineral cordierite (Mg2Al4Si5O18).
The dark areas represent hollow channels through the structure while the
white spots can be equated with regions of high atomic density. The distance
betwen the dark "spots" represents approximately 9.6 Angstroms! This figure
represents conclusive evidence for the "ordered atomic arrangement" characteristic
of minerals. Note the periodicity and the symmetry. The structure in two
dimensions, consists of an interlocking network of hexagonal (6-fold) rings.
Classification of Minerals
Minerals are classified into broad groups based on their compositional characteristics. The major groups are shown in the following table.| Mineral Group |
|
| Native Elements | C, Au, Cu, Fe |
| Oxides | MgO, Fe3O4, H2O |
| Sulfides | FeS2, PbS, CuFeS2 |
| Hydroxides | Mg(OH)2 |
| Carbonates | CaCO3, FeCO3 |
| Silicates | Mg2SiO4, KAlSi3O8, Ca2Mg5Si8O22(OH)2 |
| Sulfates | BaSO4 |
Bond Types
What is a chemical bond? We can say that there is a chemical bond between two atoms or groups of atoms when the forces acting between them are such as to lead to the formation of an aggregate as an independent molecular species.Four Main Types of Chemical Bonds
1. Covalent Bond: The most stable configuration for an atom is one in which the outer shell of electrons is completely filled. This is the atomic structure of the inert gases, and it accounts for their practically complete lack of reactivity. One way for this configuration to be achieved is by two or more atoms sharing electrons in their outer shells. In this configuration, an electron pair occupies two different orbitals simultaneously, one in each of the bound atoms. The shared electrons are confined to the two nuclei involved, i.e., they do not move to other atoms. Covalent bonds are said to be directional because the shape of the bonds are controlled by the orbitals or hybrids involved (c.f., our examples of sp3 hybrid orbitals in C and Si). Covalent bonds tend to be strong bonds and produce molecules and crystals which are electrical insulators.2. Ionic Bond: Another way for an atom to achieve a completely filled outer shell of electrons is for it to lose or gain a sufficient number of electrons. An ionic bond is one in which there has been a complete transfer of electrons from the atom of one element to the atom of the other element. The ions so formed are held together by the electrostatic attraction between oppositely charged particles. e.g., Na+ and Cl- in the mineral halite. Bonding in most of the minerals we geologists deal with is largely ionic, however, covalent bonding does occur to a lesser degree.
3. Metallic Bond: This type of chemical bond is characteristic of native metals. Metals are elements whose atoms easily lose their outer electrons. In a typical metal there are more bond sites, or empty orbitals, than there are electron pairs to fill them. Detached electrons are dispersed among the atoms in the structure and are free to move about. This electron mobility, of course, is responsible for the good electrical conductivity of metals. Metallic bonding is found in native metals and to a lesser degree in some sulfides and arsenides.
4. Van der Waals Bond: The extremely weak bonds that arise from slight imbalence of charge between two atoms or groups of atoms which otherwise have electrical neutrality. Van der Waals bonds do not play an important role in minerals, although some minerals exhibit small degrees of van der Waals character.
 These four types
of bonds provide a convenient basis for the classification of crystal structures.
It must be realized that although each type has well-defined properties,
the classification is arbitrary because bonding in most minerals is more
or less intermediate in character. For example, the Si-O bonds in silica
and in the silicate minerals are neither purely ionic nor purely covalent,
but are intermediate in character. Such intermediate bonds are typically
called Polar Covalent Bonds. In this type of intermediate
bond, although the electron pair occupies orbitals in both atoms, it spends
more time in one of them (the one with the higher electronegativity). The
directional character of these bonds also tends to be preserved. The gradational/transitional
nature of common bonds is illustrated in the diagram to the right.
These four types
of bonds provide a convenient basis for the classification of crystal structures.
It must be realized that although each type has well-defined properties,
the classification is arbitrary because bonding in most minerals is more
or less intermediate in character. For example, the Si-O bonds in silica
and in the silicate minerals are neither purely ionic nor purely covalent,
but are intermediate in character. Such intermediate bonds are typically
called Polar Covalent Bonds. In this type of intermediate
bond, although the electron pair occupies orbitals in both atoms, it spends
more time in one of them (the one with the higher electronegativity). The
directional character of these bonds also tends to be preserved. The gradational/transitional
nature of common bonds is illustrated in the diagram to the right.
The nature and the relative strengths of crystal bonds are inseparable
from implications regarding the relative sizes and distances of mutual
separation of bonded atoms. It is therefore necessary to consider the sizes
of ions. The most stable configuration of of bonded ions is achieved when
oppositely charged ions (e.g., Na+ and Cl-) are as close together as possible
without overlapping.
The SiO4 Tetrahedron as the Building Block for Silicate Minerals
As oxygen is the most abundant element in the earth's crust, most of the rock-forming minerals of interest to geologists are composed of various cations bonded to oxygen. The ionic radius of oxygen is 1.36 Angstroms, and hence it is very large compared to the cations with which it commonly bonds. Behind oxygen in relative abundance in the crust are Si (ionic radius = 0.26 Angstroms) and Al. Si is especially important as silicon bonded to oxygen forms the basic building block of all silicate minerals. In terms of size, the most stable geometric arrangement for the small Si cation is to fit in the void created at the center of 4 oxygen anions arranged in the shape of a tetrahedron. In addition to size considerations, the electron configuration of Silicon is such that the four valence electrons in the 3S and 3P orbitals form polar covalent bonds known as SP3 orbitals. These orbitals are directional and point away from the Si ion toward the corners of a tetrahedron. Three views of the SiO4 tetrahedron are shown in the following figure:
A. Silicon has 4 electrons in its outer energy-level
shell (M). Each of the 4 elactrons is shared with one Oxygen, and
each oxygen in turn shares one oxygen with silicon.
B. Tetrahedral-shaped silicate molecule with oxygens
touching each other in natural positions. Silicon occupies the central
void between the 4 oxygens.
C. Expanded view of the molecule showing the oxygens
at the corners of the tetrahedron with bonds to the silicon at the center
of the tetrahedron.
NB. The bonding of 4 oxygens and 1 silicon does not acheive charge neutrality. The molecule, in fact, has a net charge of -4. The fundamental building block of the silicate minerals is therefore [SiO4]-4.
Different groups of silicate minerals arise from the several ways in which the [SiO4]-4 tetrahedra can be linked together to form silicate crystal structures. As an example, the olivine group of minerals is formed by bonding isolated [SiO4]-4 tetrahedra to two divalent cations such as Mg2+ or Fe2+ . This achieves both charge balance and results in a three-dimensional array of isolated [SiO4]-4 tetrahedra linked by the metal cations.
Another way in which [SiO4]-4 tetrahedra can be linked together is for the tetrahedra to share some or all of their corner oxygens with adjacent tetrahedra. This is an example of the process known as polymerization. For example, the mineral group pyroxene, consists of [SiO4]-4 tetrahedra linked together to form single chains. In this structure, each tetrahedron shares two of its 4 oxygens with adjacent tetrahedra. Consequently, the building block formula is [SiO3]-2 . These single chains can then be linked together by bonding with one divalent cation such as Mg2+ or Fe2+ , to yield the formula (Mg, Fe) SiO3 .
The main groups of silicate minerals that result from polymerization of [SiO4]-4 tetrahedra are shown in the following figure/table:
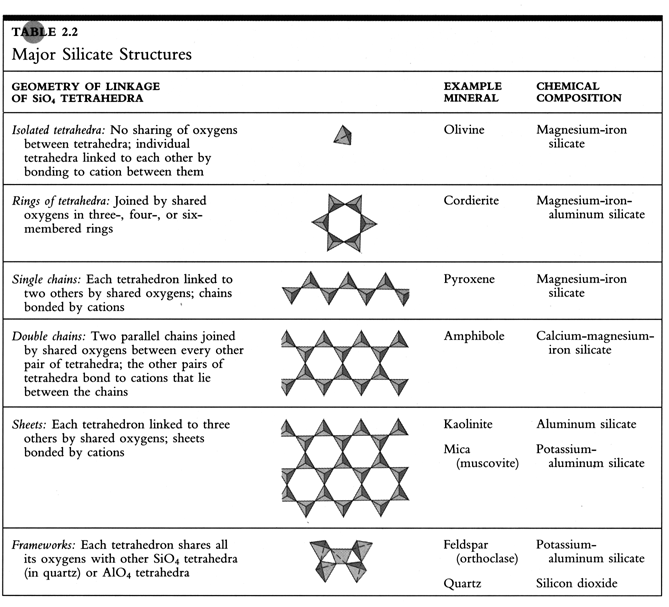
The three-dimensional framework minerals are especially important as minerals in this structural group are the most abundant minerals in the continental crust. Quartz is the simplest of this group; it results from sharing of all four of the corner oxygens, resulting in a formula of SiO2 with no need for additional cations to balance charge.
The feldspars arise by substituting Al3+ for Si4+ in some of the tetrahedra. If, for example, we substitute one Al in every four tetrahedra, we obtain a molecule of [AlSi3O8]-1. The charge on this molecule can be balanced by bonding with monovalent cations such as Na+ or K+ . This produces minerals such as alkali feldspar, KAlSi3O8 or NaAlSi3O8 .
We do not expect you to become expert mineralogists from this course,
but do hope that this series of lectures has provided you with a better
understanding of the basic structures, and the ways in which crystal structure
plays an important role in determining many of the physical properties
displayed by minerals. These include properties such as cleavage,
hardness, habit, symmetry, color and many more.
