Lecture 9
Introduction to Igneous Petrology
Introduction
Igneous rocks form by cooling and
crystallization of hot silicate liquids.
They are usually divided into two groups:
-
intrusive rocks
(like granite) that cool and crystallize at depth, and
-
extrusive rocks
(like basalt) that erupt onto the Earth’s surface
Igneous
rocks comprise the bulk of oceanic crust and much of the continental crust; the
mantle is also made of rocks generally deemed igneous.
Terminology
magma – silicate liquid +/- crystals +/-
dissolved volatiles
lava
– silicate liquid
+/- crystals (lava loses its volatiles during ascent and eruption on the
Earth’s surface)
Melting
and Crystallization
The mantle is largely solid, thus
magma must form by melting of the mantle.
There are three main ways to melt the mantle:
1.
increase temperature
2.
decrease pressure
3.
add H2O

What types
of melting occur in which tectonic environments?
1. Divergent
boundaries
(mid-ocean ridges) produce melts primarily by decompression … these melts are
basaltic and either erupt as pillow basalts or sheet flows, or form intrusive dikes, sills, and gabbros.
 2. Hot spots (like Hawaii), as their name
suggests, produce melts primarily by heating; these melts are also of basaltic
composition. Hawaiian basalts
erupt primarily as very fluid lava flows that build large shield volcanoes with
low slopes.
2. Hot spots (like Hawaii), as their name
suggests, produce melts primarily by heating; these melts are also of basaltic
composition. Hawaiian basalts
erupt primarily as very fluid lava flows that build large shield volcanoes with
low slopes.

3. Convergent
boundaries (subduction
zones) recycle wet sediment and basalt into the mantle, thus melts produced in
this environment are created largely by fluxing of the mantle with H2O. The melts produced are variable in
composition, but are generally basalt or andesite.
Melting
and phase diagrams
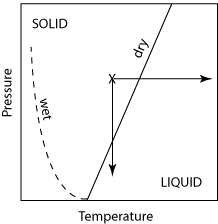
To understand the concept of melting
and crystallization of a multicomponent system we need to understand something
about phase diagrams. [explained using
handouts in class; see also Box 5.5 in your text]. BOTTOM LINE: when you melt a multicomponent system, the
initial melt has a different composition than the bulk solid, and that
composition will change with increased amounts of melting.
THEREFORE: melt composition depends not only on
the bulk composition of the solid but also on the amount of melting!
Petrology
of the Mantle
1.
Seismology and mineralogy
Seismic studies define changes in
the physical properties of mantle rock with depth. Important boundaries include:
a)
the Moho – boundary
between low density felsic and mafic rocks of the crust and high density
ultramafic rocks of the mantle
b)
lithosphere-asthenosphere – boundary between the rigid outer layer of the Earth and a mechanically
weak layer that acts like a viscous fluid
c)
400 km discontinuity –
increase in seismic velocity (density) caused by a change in olivine structure
to that of spinel
d)
670 km discontinuity
– change in spinel structure of olivine to that of perovskite
2.
Composition of the mantle
We have samples of the upper mantle
in the form of xenoliths (mantle fragments), dredged and drilled samples from
oceanic fracture zones, and ophiolites, slabs
of upper mantle thrust onto the edge of continents at old suture zones.
The upper
mantle is peridotite (olivine-bearing).

(from
Winter, 2001)
Basalt forms by melting of lherzolite,
leaving a residue of harzburgite + dunite.
 Although olivine and pyroxene are
the dominant phases in the mantle, minor aluminum-bearing phases can tell us a
lot about the P-T conditions of the mantle. Aluminous phases include plagioclase (low pressure), spinel
(intermediate pressure) and garnet (high pressure; diagram from Winter, 2001).
Although olivine and pyroxene are
the dominant phases in the mantle, minor aluminum-bearing phases can tell us a
lot about the P-T conditions of the mantle. Aluminous phases include plagioclase (low pressure), spinel
(intermediate pressure) and garnet (high pressure; diagram from Winter, 2001).
We can use
this diagram to explain why plagioclase-bearing xenoliths are common in oceanic
regions, and garnet-beraing xenoliths are restricted to beneath the continents
(where the pressures are high and the geothermal gradient lower).
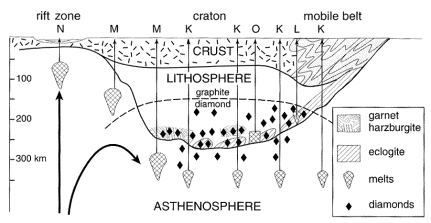
Also unique
to continental (kimberlite) xenoliths are diamonds … WHY? Formation of diamons from graphite requires high pressures
at moderate temperatures, and even higher pressures at high temperatures. The low geotherm under old cratons thus
decreases the pressure of this transition, and allows diamonds to be preserved
and carried to the surface (diagram from Winter, 2001):