Lecture 16 Ð Metamorphic Rocks 3
 Last time we talk about mineral
reactions that could be used as geothermometers and as geobarometers to constrain the pressure and temperaure of metamorphism. Garnets are chemically slow to react so
that, once formed, the composition of garnet can be Òlocked inÓ, even though
the composition of later-formed garnet may be very different. Thus natural garnets are commonly
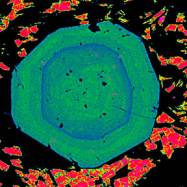
strongly zoned in Mg, Fe, Ca, and Mn, as shown by the image to the right (a
garnet from n. Idaho). This is a
false-color image showing the concentration of Ca in the mineral. Red represents high Ca, blue-green show
intermediate values. Look closely
at the blue zones, which suggest at least two, and probably three, stages of
garnet growth.
Last time we talk about mineral
reactions that could be used as geothermometers and as geobarometers to constrain the pressure and temperaure of metamorphism. Garnets are chemically slow to react so
that, once formed, the composition of garnet can be Òlocked inÓ, even though
the composition of later-formed garnet may be very different. Thus natural garnets are commonly
strongly zoned in Mg, Fe, Ca, and Mn, as shown by the image to the right (a
garnet from n. Idaho). This is a
false-color image showing the concentration of Ca in the mineral. Red represents high Ca, blue-green show
intermediate values. Look closely
at the blue zones, which suggest at least two, and probably three, stages of
garnet growth.
We can then
use these chemical zones to determine the P-T path over which that garnet
grew.
Next step
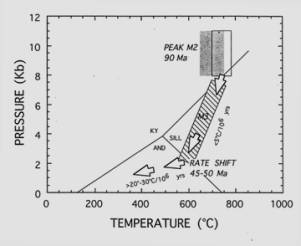
is to determine over what time period this growth happened. The figure below
shows the radiometric age of peak metamorphism in n. Idaho related to the Idaho
batholith. ,Note the rapid uplift
of this region between 90 and 50 Ma, during which time the decompression was
nearly isothermal:
This sort
of uplift (decompression) path requires that the rocks be heated while they
were being uplifted. The source of
heat in this area can only be the Idaho batholith, thus batholith emplacement
and metamorphism were intimately related.
Additionally, the high temperatures of metamorphism are sufficient to
cause dehydration melting, thus providing an explanation of the source of the
batholith. Once formed, the
batholith rose rapidly (since it was hot and buoyant), carrying the rocks along
with it.
The
thermodynamics of reactions
The question was raised about
equilibrium, and how we know it has been achieved. One of the most important indications of equilibrium is that
the mineral assemblage is stable, particularly as determined by GibbsÕ phase
rule (p + f = C + 2) which tells us the number of phases that are allowed to
exist at equilibrium for a given number of components, when the only intensive
variables that can
change are pressure and temperature.
F = C system
is divariant (common)
F < C occurs
when systems exhibit solid solution
F> C either
P or T is fixed, OR we didnÕt pick the right number of components (a common problem!),
OR the system is not at equilibrium (e.g., partially completed retrograde
reactions)
Last time
we looked at the simple one-component system Al2SiO5. Now letÕs look at a more complicated 3-component system
Al2O3 Ð SiO2 Ð H2O (Fig. 7.13 in your book). The reactions shown are univariant (degree of freedom = 1),
and thus involve four phases:
Al2Si2O5(OH)4
+ 2SiO2 = Al2Si4O10(OH)2
+ H2O
kaolinite
+
2quartz = pyrophyllite +
water (vapor)
This is a
low temperature dehydration reaction.
NOTE: this reaction does NOT limit the stability field of quartz IF
kaolinite is not present!
Al2Si4O10(OH)2
= Al2SiO5 + 3SiO2 + H2O
pyrophyllite = kyanite + 3quartz + water (vapor)
Pyrophyllite
is stable over only about 100ûC, at which point it dehydrates to form the
anhydrous phases kyanite and quartz.
In fact, in this system the only phases that are stable over 450ûC are
corundum, quartz, and an aluminosilicate.
We can also
use chemographic diagrams to look at phase relations graphicallyÉ. [EX]
LetÕs
say that the rocks in our area exist of the following mineral assemblages:
x-xy-x2z
xy-xyz-x2z
xy-xyz-y
xyz-z-x2z
y-z-xyz
Minerals
that coexist are connected by tie lines..any specific assemblage of minerals
represents a bulk rock compositionÉ
The Gibbs
free energy of a
mineral is a way of expressing the relative stability of that mineral in a
specific P,T,X space. The Gibbs
free energy of a reaction is the difference between the Gibbs free energy of
the products and of the reactants.
Consider
the equilibrium between calcite and its polymorph, aragonite (Fig. 7.16). the reaction relating the two is simply
CaCO3
(cc) = CaCO3 (ar)
The Gibbs
energy of the reaction ÆG
= Garagonite - Gcalcite
If Garagonite
< Gcalcite, then ÆG is negative and aragonite is stable. If Garagonite > Gcalcite
then ÆG is positive and calcite is stable. Calcite and aragonite can coexist only when ÆG = 0; the P-T
conditions at which this is true are defined by the phase boundary.
How do we
determine ÆG? There are
thermodynamic tables that give the Gibbs free energy of formation (ÆGûf) for most
minerals, given in J/mol (or Kcal/mol).
This number refers to the amount of energy released (in joules or Kcal)
when pure elements react to produce one mole of the mineral in question. Because ÆGûf is negative
(exothermic, that is, energy is released), calcite is more stable than the
individual elements. We can use
the formation energy to determine the energy of reaction:
ÆGrxn
= ÆGf(aragonite) - ÆGf(calcite)
So far we
havenÕt talked about the effect of P,T.
Gibbs free energy varies
with P,T (which is why phase boundaries vary with P,T). The variations depend on internal
energy (E, also called enthalpy), molar volume (V), and molar entropy (S) as
G
= E + PV Ð TS
Similarly,
we can write this equation in terms of reactions:
ÆGrxn
= ÆErxn + PÆVrxn Ð TÆSrxn
where in
each case, the change of the parameter with reaction is the difference between
the products and reactants. LetÕs
think about what these equations mean.
If PÆV is large (that is, high pressure and/or large molar volumes) then
ÆG will be large and the mineral will be unstable. Therefore, at high pressures, samples with low molar volume
(high densities) will be most stable.
Similarly, at high T, high entropy samples are most stable (also
decreases G).
If a
reaction is at equilibrium, then
ÆGrxn
= 0 = ÆErxn + PÆVrxn Ð TÆSrxn
From this
equation we can derive an important equation known as the Clausius-Clapeyron
equation, used to
calculate the slope of a reaction on a P-T diagram:
slope
= dP/dT = ÆSrxn/ÆVrxn
For most
solid-solid reactions, S and V vary little so phase boundaries are straight
lines. In contrast, dehydration
and decarbonation reactions plot as curves because the volumes and entropies of
fluids like H2O and CO2 vary greatly with P and T, leading to great variations
in ÆV and ÆS.
In theory,
we could use this approach to predict phase diagrams for systems of any
composition. In practice, things
get complicated, particularly for minerals that form solid solutions. Additionally, we donÕt have good thermodynamic data for all
mineral species.
The Rock
Cycle
Over the past several weeks, we have
seen that Earth material can start as igneous rock, then be weathered, eroded,
transported and deposited as sediment.
This sediment can then be lithified and converted to a sedimentary rock
which may be subsequently metamorphosed.
If metamorphism is sufficiently intense, the rock can melt and start the
cycle all over again. This
sequence is known as the rock cycle (or recycle!).