Sedimentary Rocks
Introduction
Once exposed to the EarthÕs surface,
all rocks are subjected to processes of erosion, transportation and
depositionÉ Thus sediment becomes sedimentary rock. Sedimentary rocks are classified on the basis of the nature
of the sediment that they were formed from. Sedimentary rocks are usually divided into two main groups:
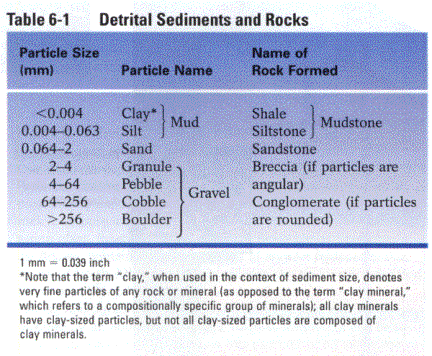
DETRITAL Š named for the size of the detrital particles
(e.g., sandstone, siltstone, mudstone)
CHEMICAL Š named on the basis of chemical composition
(limestone, chert, evaporates); sometimes biogenic rocks are classified separatelyÉ
Detrital
(clastic) sediments
Involves erosion, transportation and
deposition by moving water.
Requires energy thresholds to transport particles of different sizes,
therefore water-transported detrital rocks are often well sorted by grain size.
Conversion
of unconsolidated sediment into sedimentary rock is called diagenesis or lithification Š achieved by both compaction and
cementation. Typical cements are
calcite and quartz. Diagenesis is
hastened by circulation of heated fluids, such as those given off from
compaction of buried sediments in deep basins.
Weathering
Weathering forces may be either
mechanical or erosional. Mechanical
weathering, which produces detritus, is much less significant than chemical
weatheringÉ Chemical weathering
produces dissolved material (especially alkalis and alkaline earths) and
leftover rock (typically quartz, clay, kspar, garnet, zircon, rutile). Notice that both quartz and feldspar
were end members in BowenÕs reaction series É thus BRS can be used to indicate
the order of increasing stability of mineralÕs at the EarthÕs surface (with qz
and fsp some of the most stable, and olivine the least stable).
Hydrolysis
reactions Š reactions that involve H2O. Reactions are aided by the fact that
natural rainwater is slightly acidic, thanks to the interaction of CO2
and H2O:
CO2(gas)
+ H2O(liquid) ¨¸ H2CO3 (carbonic acid)
H2CO3
¨¸ H+ + HCO3-
Similarly,
sulfuric acid (H2SO4) can form in sulfur-rich
environments .. this is a much stronger acid than carbonic acid.
Weathering
of calcite
Calcite weathers by a simple dissolution reaction: CaCO3 ¨¸ Ca2+ + CO32-
The resulting carbonate ion dissolved in solution combines
with H+ to form carbonic acid.
As a result, the concentration of H+ is decreased, increasing the pH and
ŅbufferingÓ the acidity of the water.
Weathering
of pyroxene and olivine
The weathering of px and ol involves
the breakup of the silicate structure to release Fe, which is then oxidized.
EX: 4FeSiO3
+ O2 + H2O ¨¸ FeO(OH)(solid) + 4SiO2
(solution)
The
hydrated Fe mineral is called limonite, a clay-like mineral that makes Fe-rich
soils red.
Weathering
of feldspar
Weathering of feldspar also involves
the reaction of the crystal with acidic water:
EX: 2KAlSi3O8
+ 2H+ ¨¸ Al2SiO5(OH)4
+ 4SiO2 + 2K+
where the
hydrous aluminous phase is the clay mineral kaolinite. Other clay minerals include smectite (a swelling clay) and illite
(a mica-like clay). In general,
clay minerals are sheet silicates that tend to be very fine-grained (hence the
use of the term clay for both a class of minerals and a particle size). The end member of chemical weathering
of feldspars is residual aluminum oxides and hydroxides, known as laterites (if not lithified) or bauxites, an important source of aluminum.
Clays are widely used in industry, arts
& ceramics. They are also a
fundamental component of soils (weÕll come back to this at the end of term).
Chemical
sedimentary rocks
Chemical sedimentary rocks are those
that precipitate from solutions.
The ease of precipitation is inversely proportional to the solubility of
the mineral in water. Thus silica
often precipitates early in an evaporation sequence to form thick chert beds (most common in Precambrian
rocks).
Carbonate minerals such as calcite are somewhat more soluble, and
thus require slightly more evaporation.
Carbonate precipitation may be in the form of fine-grained limestone
muds, which form fine-grained limestone called micrite. Many carbonate rocks contain clastic material and/or
fossils.
Salts such as gypsum (CaSO4.2H2O), halite and sylvite
are highly soluble so that their chemical components are common dissolved
species in water. These components
precipitate out as evaporites in enclosed inland basins.
Other chemical sedimentary rocks include phosphorites and
iron formations. the latter are mostly Precambrian in age, and composed of Fe
oxides and hydroxides, magnetite, or Fe-carbonate (siderite, FeCO3). Probably formed in shallow marine
conditions (like present day Mn
nodules).
Diagenesis
Diagenesis refers to chemical,
mineralogical, or textural changes that occur in sediments or sedimentary rocks
after deposition, but before metamorphism. Includes compaction, recrystallization, and leaching
(dissolution).
Oceans Š
the final repository
There are both inputs to and outputs
from ocean basins.
Inputs: detrital
solids carried by rivers
dissolved
ions in river water
Outputs: detrital sediments
chemical
precipitates
biological
sediments
Dissolved
input to the ocean is balanced by precipitation. The average length of time that a particular element remains
dissolved in sea water is the residence time, and can be calculated from
(total amt. of element in sea water) / (rate of input to
oceans)
RT
= A/(dA/dt)
Most common
elements (Ca, Mg, Na, K, S) have residence times ~ 1-10 million years.
Importance: needed to look at
potential residence time for toxic chemicals, or CO2 (related to
climate change).
The
chemical reactions that cause minerals to precipitate are controlled by
physical parameters such as temperature and concentration of dissolved species, and by partial
pressure of gaseous components involved in the reaction sequence.
The fate
of CO2
Increases in atmospheric CO2
has a well documented impact on global climate. Increased levels of atmospheric CO2 will also
result in more acidic rainwater and will thus increase rates of chemical
weathering. In turn, increased
weathering rates will result in an increase in the input of dissolved materials
to the oceans.
Oceanic
processes that reduce atmospheric CO2:
-
photosynthesis by blue-green algae in the euphotic (light-penetrating) zone
-
precipitation of CaCO3
Thus there
the oceans have a limited ability to buffer atmospheric CO2. Which raises the question Š is there
evidence that the ocean has changed composition through time?
YES! The best evidence is the extent of
banded iron formations in the Precambrian. (UP Michigan), formations that resulted from precipitation
of Fe-bearing minerals (e.g. FeCO3) from seawater. Currently, the ocean has very low
levels of dissolved Fe. However,
in the Precambrian, atmospheric O levels were much lower, meaning that Fe
existed at the EarthÕs surface in a reduced, rather than oxidized, state. As Fe2+, iron was soluble in
the oceans and accumulated in considerable amount. As the atmosphere became more oxidizing, nearly all of the
dissolved iron was precipitated to form the large iron formations.
The role
of biological sediments:
Most
important biological sediment is coal É forms from the diagenesis of peat and
other organic matter.