
Hoffman's acetylated salicylic acid was kinder to the stomach of his father -- and it also created wealth for his employer, the Bayer Corporation. In the years since Hoffman's work, scientists have come to understand the molecular basis for aspirin's action. Much of this is due to the work of Sir John R. Vane in England. Basically, Sir John and others found that aspirin inhibits a single enzyme involved in an early step of the conversion of arachidonic acid to prostaglandins (Reference to prostaglandin pathway). The enzyme has several names including prostaglandin-H2 synthase (PGHS) or cyclooxygenase (COX). It was later discovered that there are at least two forms of COX: COX-1 and COX-2. COX-1 was found to be important in the protection of the lining of the stomach, as well as other important functions. COX-2, unlike COX-1, was not always present in the cells of the body (it was induced, not constitutive), but appeared under certain stimuli. Once it was made in a cell, it was involved in processes that resulted in inflammation, pain, and fever.
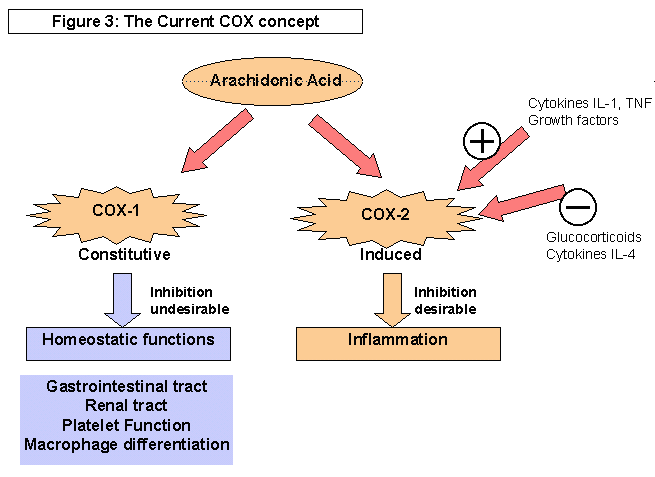
Graphic from: http://www.arthritis.co.za/cox.html
This got people thinking about the possibility of making a drug that would shut down COX-2 while leaving COX-1 alone. (Hoffman's dad would have danced a jig!). While the various non-steroidal anti-inflammatories had different selectivities toward COX-1 and COX-2, none stood out as completely selective.
To design a drug that would shut down COX-2 and leave COX-1 alone, researchers needed the 3-dimensional structure of both enzymes. X-ray crystallography, in which x-rays are scattered by a crystal of the protein and the scattering pattern analyzed to give the structure, provided the answers. Below is the COX-1 enzyme:
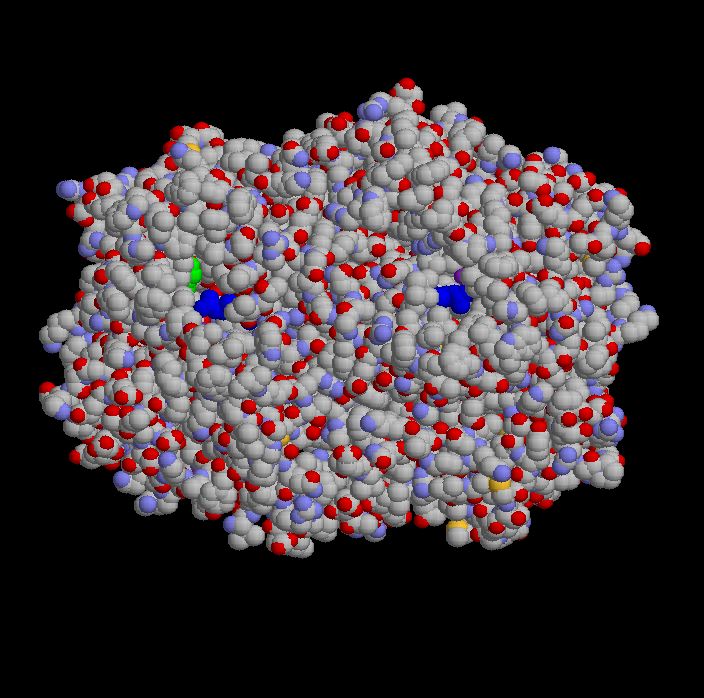
COX-1 is actually composed of two identical chains (subunits), seen here as the left and right sides of the picture. I've colored dark blue the amino acids arginine that sit at the opening in each subunit into which aspirin fits. (This is the active site for one of the two activities of the enzyme). The green you see on the left is a serine residue (another amino acid) that is chemically modified, acetylated, when aspirin enters the active site. Since arachidonic acid normally enters this same active site to be worked on by the enzyme, the acetylation of serine blocks its access to the channel and irreversibly inhibits COX.
Here's a picture of COX-1 showing only one of the two identical subunits. I've
colored the amino acids that help form the active site as follows: arginine
120 (it's the 120th amino acid in the protein) is dark blue; tyrosine 385 is
yellow; and the heme group (a large, planar molecule with an iron in its
center) is red. You are looking down the "hole" that aspirin--or any other
NSAID fits into when it
inhibits COX-1:
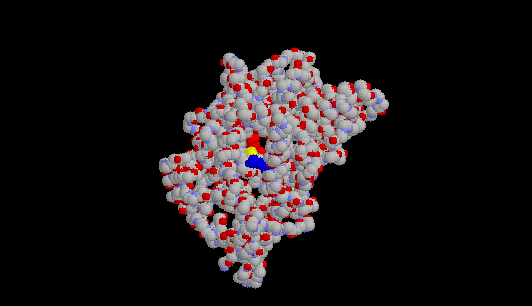
Here's another view of the active site of COX-1, with salicylic acid sitting in that channel or "hole."
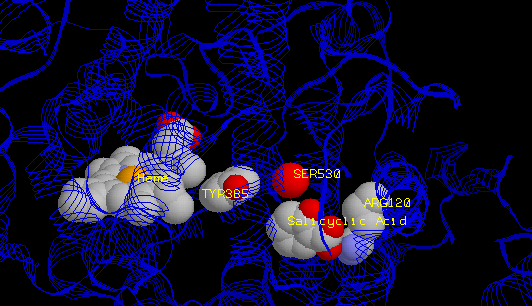
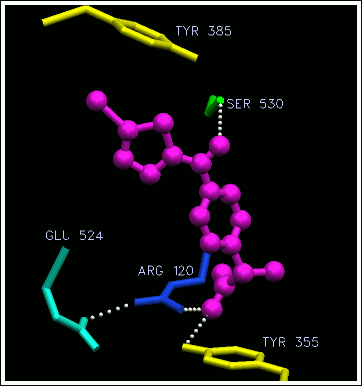
When aspirin binds into the active site, the acetyl group gets transferred to serine 530 (the red sphere is its oxygen atom). This effectively destroys any activity of COX. Normally, it just sits around inactive until it gets recycled (broken back into separate amino acids). Once you've taken aspirin, only newly made COX is active.
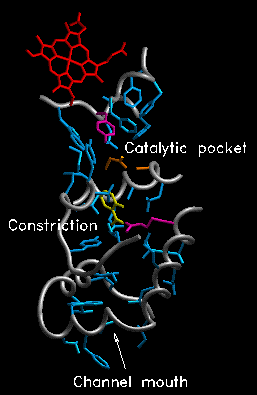
Many other NSAIDs bind in the same part of the active site channel (right), as illustrated by suprofen (left: Biochemistry 35:7330-7340 (1996)); they, however, do NOT covalently modify enzyme. They just sit in the active site channel and keep COX from working. (These two images from the Loll lab--he has a new article in J. Mol. Biol. 335:503-518, if you're interested). The image on the left shows a constriction that is present in COX-1, but not COX-2. The COX-2 binding site is larger due to the substitution of a single amino acid (valine for an isoleucine at position 523), and has a secondary internal pocket off the inhibitor binding site not seen in COX- 1. Thus, COX-2 specific inhibitors are too big to fit into the COX-1 channel.
Here are some URL's for more information (not a comprehensive list!):