Lecture 6
Crystal growth, Physical
properties of minerals
Crystallization
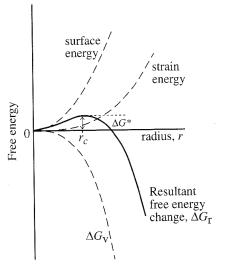
Crystallization
involves nucleation of a “seed” crystal and subsequent growth of that
crystal. Nucleation involves
competition between the supersaturation driving crystallization and the surface
energy created by formation of a new phase. For this reason, high supersaturations (a large driving
force) promotes nucleation. In
contrast, once nuclei exist, they may grow at smaller supersaturations.
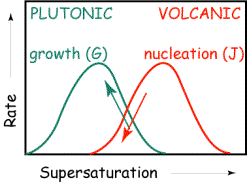 The figure below illustrates the
different supersaturation regimes anticipated for different locations of magma
cooling. Slow cooling (low
supersaturation) of rocks within the Earth’s crust is often invoked to explain
the lower number density, but
larger size, of crystals in plutonic rocks relative to volcanic rocks
that cool on the Earth’s surface.
The figure below illustrates the
different supersaturation regimes anticipated for different locations of magma
cooling. Slow cooling (low
supersaturation) of rocks within the Earth’s crust is often invoked to explain
the lower number density, but
larger size, of crystals in plutonic rocks relative to volcanic rocks
that cool on the Earth’s surface.
Differences
in nucleation and growth behavior can also explain the difference between the
salt and alum crystals that you grew in lab….
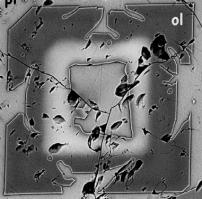
Crystal growth
Processes
of crystal growth aren’t perfect… for example, crystals that grow rapidly may
develop skeletal or dendritic forms.
Such a crystal is shown in the photo on the right. This is a scanning electron microscope
image where backscattered electrons are collected to yield an image that tells
us about composition. You can see
many different features in this image.
First, the crystal appears to have a hole in the middle … this is the
texture that we call skeletal. Second, note
that the structures at the corners of the crystals are “decorated” with the
onset of dendritic
overgrowths. Finally, note that
the crystal is zoned in gray scale, which represents compositional zoning
(discussed below).
And most
crystals have some sort of imperfection (many of which are diagnostic of that
crystal). Which leads to a
discussion of:
Crystal
imperfections - defects
Defects
important in that they increase crystal reactivity ...
Point
defects
All
crystals above absolute zero contain some defects ... increases energy of
system, thus more at high temperatures
1.
Impurity defect –
results from the presence of a foreign atom, either replacing one normally in
the structure or filling a “vacancy”.
2. Paired vacancies Anion
vacancies are regions where there is more positive charge - may trap nearby
electron ... transitions between energy levels may be invisible range - color
center For this reason, can induce colors
using radiation (Pleichroic halos
around zircon inclusions) Vacancies are important for process of diffusion,
that is, moving ions through the crystal structure.
3. Line defects - happen when rock is stressed. Most easily understood with reference to simple cubic
lattice. dislocations - extra plane of atoms.
edge dislocation – occur when a plane of atoms in a structure terminates at a
line in the crystal’s interior
screw dislocation - caused by displacement of part of crystal structure one
translation unit relative to another such that the displacement terminates
along a line perpendicular to growing face.
4. Stacking faults – may separate layers that are out of order
Crystal
imperfections – zoning
 Compositional zoning occurs when
different parts of a mineral have different compositions (through various
substitution mechanisms). This is
illustrated nicely in the olivine picture above, where the dark gray part of
the crystal is enriched in Mg, and the bright part of the crystal is rich in
Fe.
Compositional zoning occurs when
different parts of a mineral have different compositions (through various
substitution mechanisms). This is
illustrated nicely in the olivine picture above, where the dark gray part of
the crystal is enriched in Mg, and the bright part of the crystal is rich in
Fe.
Compositional
zoning is particularly common (and diagnostic) in plagioclase. The crystal shown on the left shows
several different types of zoning, including
normal
zoning (the general
outward trend from brighter, Ca-rich plagioclase to darker, Na-rich
plagioclase)
oscillatory
zoning (the fine
scale structure that oscillates between slightly darker and slightly lighter
shades of gray)
sector
zoning (which is
illustrated by the faint diagonal bands that open outward from the crystal’s
interior
Crystal
imperfections - twinning
Twins
result when different domains of a crystal structure have different
orientations. They share atoms
along a surface –
typically a composition plane. You can
think of these as mistakes in where the next plane of atoms is placed (think of
stacking the clear plastic spheres …
Descriptions
of types of twinning:
simple
twins – if composed
of only two parts, that is, if the twins can reflect across a single plane
multiple
twins – refers to
twins of multiple orientations
penetration
twins – describes
the growth condition when two or more parts of a crystal appear to penetrate
each other.
Most common
(and distinctive) are the twinning habits of feldspars. Plagioclase feldspar is triclinic, thus
there are no planes of symmetry to control twin planes. Twinning in plagioclase consists of
numerous repeated twins, called polysynthetic twins. Most spectacular when viewed in thin section.
Physical properties of
minerals
We’ll look
at two different aspects of physical properties – those that are important for
diagnostic identification of minerals (mostly “scalar”), and those that dictate
the physical behavior of minerals (those that often show directionality, that
is they are “vector” properties).
HAND
SPECIMEN PROPERTIES
As these
properties are best learned in lab, I will just present an overview in class.
1.
Appearance

 LUSTER – general appearance or sheen … examples include metallic, vitreous,
adamantine (diamond-like).
Metallic luster is the result of near-complete reflection of light by
the mineral surface. The
adamantine luster of diamond is a consequence of its high index of refraction
LUSTER – general appearance or sheen … examples include metallic, vitreous,
adamantine (diamond-like).
Metallic luster is the result of near-complete reflection of light by
the mineral surface. The
adamantine luster of diamond is a consequence of its high index of refraction
 DIAPHENITY
– refers to a mineral’s ability to transmit light (transparent, translucent,
opaque). Most opaque minerals have
metallic luster.
DIAPHENITY
– refers to a mineral’s ability to transmit light (transparent, translucent,
opaque). Most opaque minerals have
metallic luster.
COLOR
– often useful for quick ID (particularly when color is distinctive), but can
be very misleading. Color is controlled
by “chromophores” and is a consequence of the interaction of light with
electrons in the crystal.
Allochromatic minerals have color caused by
elements that are present in trace amounts, like the Cr that causes the green
color of beryl to the right (emerald), or the Ti that gives corundum the blue
that we call sapphire.
In
contrast, idiochromatic minerals have color as an intrinsic property, sometimes on that  changes with solid solution
composition, and thus may be diagnostic not only of mineral type but also end
member composition (as in
changes with solid solution
composition, and thus may be diagnostic not only of mineral type but also end
member composition (as in  garnet). Examples of idiochromatic minerals include Cu-bearing
minerals (which are typically blue or green) and Mn-bearing minerals, which are
typically pink. Color can also be
created by electron vacancies to form “color centers” (particularly common in
fluorite).
garnet). Examples of idiochromatic minerals include Cu-bearing
minerals (which are typically blue or green) and Mn-bearing minerals, which are
typically pink. Color can also be
created by electron vacancies to form “color centers” (particularly common in
fluorite).
STREAK
– the color of finely powdered mineral; useful for distinguishing oxides and
sulfides
LUMINESCENCE
– any emission of light that is not the direct result of incandescence;
includes properties such as fluorescence and phosphorescence. Luminescence of minerals is another property that may be
controlled by trace amounts of an element.
COLOR
PLAY – refers to properties of light scattering,  as seen in the “star sapphire” in
the picture. In this case the
“star” of light is created by light scattering from small inclusions that are
arranged along the three principle crystallographic directions.
as seen in the “star sapphire” in
the picture. In this case the
“star” of light is created by light scattering from small inclusions that are
arranged along the three principle crystallographic directions.
 Other
examples of color play include the iridescence that is characteristic of
labradorite; here the scattering is the result of very fine-scale exsolution.
Other
examples of color play include the iridescence that is characteristic of
labradorite; here the scattering is the result of very fine-scale exsolution.
Opalescence
is probably one of the best examples of color play – opalescence is the result
of silica precipitation as tiny spherical bodies that are able to scatter
light.
2.
Crystal shape

 Called crystal “habit” – the
appearance of minerals, either as single crystals or as aggregates; includes
terms such as the fibrous growth of cerussite (Pb carbonate) crystals to the
left, or the botryoidal habit of smithsonite (Zn carbonate) to the right.
Called crystal “habit” – the
appearance of minerals, either as single crystals or as aggregates; includes
terms such as the fibrous growth of cerussite (Pb carbonate) crystals to the
left, or the botryoidal habit of smithsonite (Zn carbonate) to the right.
3.
Strength – related primarily to bonding
TENACITY – cohesiveness, or resistance to breaking. Terms to describe tenacity include
brittle (ionic bonding); malleable (metallic bonding), flexible (characteristic
of sheet silicates like mica)
CLEAVAGE, FRACTURE, PARTING – reaction of crystal (strain)
to an external force (stress).
“Cleavage” is the tendency of minerals to break along certain planes
(EX: graphite). When minerals
break along planes of weaknes they have “parting”; weakness may be twinning,
pressure solution. When minerals
do not have a dominant plane of weakness they “fracture” in patterns that may
be described as “conchoidal”, “fibrous”, “hackly”.
HARDNESS – resistance of a smooth surface to
scratching. Hardness is probably a
consequence of weakest bond in structure.
4.
Density (specific gravity)
Ratio of the weight of a substance
and the weight of an equal volume of water. Determined as
![]()
Specific
gravity (the ratio) can be measured by a Jolly balance; density requires a
pycnometer. The density can be
calculated from the mineral formula if you know the dimensions of the unit cell
and the number of formula units per unit cell.
5.
Magnetism
Magnetite and pyrrhotite are the
only common minerals with a magnetic signature.
ANISOTROPY
AND PHYSICAL PROPERTIES
Many
physical properties are anisotropic, such that their magnitude depends on the direction in the
crystal. An easy way to picture
this is by a mechanical analogue with springs of different stiffness in
different directions… net displacement is the result of the vector sum of the
components, thus the direction of displacement is not necessarily the same as
the direction of the applied force.
DIRECTIONAL
PROPERTIES
thermal
conductivity relateds
heat flow to temperature gradient
electrical
conductivity relates
electrical current density to electric field
diffusivity
relates atomic flux
to concentration gradient
elastic
properties relate
strain (extent of deformation) to applied stress
seismic
properties relate
to velocity of seismic wave propagation (related to density, rigidity, bulk
modulus)
optical
properties relate
to refractive index variations
Examples: calcite shows double refraction
ulexite is a natural fiber optic
Each of
these properties is controlled by the crystal structure, such that
•
the directional variation in the value of a physical property must be
consistent with the point group symmetry of the crystal
•
since physical properties can always be broken into three mutually
perpendicular components, the symmetry of physical properties may be greater
than the symmetry of the crystal itself
Physical
properties may be
isotropic – uniform in all directions
(isometric crystals)
uniaxial – similar in two directions and
different in the third (hexagonal and tetragonal crystals)
biaxial – different in all three directions
(orthorhombic, monoclinic, trigonal crystals)