Lecture 5
Classification of Minerals;
Crystal growth
Review
from last time - Paulings Rules:
1. The Coordination (radius ratio)
Principle – a
coordination polyhedron of anions surrounds each cation. The cation-anion distance is determined
by the sum of the cation and anion radii and the number of anions coordinating
with the cation is determined by the relative size of the cation and anion.
2.
Electrostatic Valency Principle – in a stable ionic structure, the total strength of the
valency bonds that reach an anion from all neighboring cations is equal to the
charge of the anion.
3.
Sharing of Polyhedral Elements I – the existence of edges (and particularly faces) common to
coordination polyhedra decreases the stability of ionic structures
4.
Sharing of Polyhedral Elements II – in a crystal containing different cations, those with
large valence and small coordination number tend not to share polyhedral
elements with each other.
5.
Principle of Parsimony – the number of essentially different kinds of constituents in a crystal
tends to be small.
Chemical
Variation in Minerals
Pauling’s
rules can be used to explain not only crystal structure, but also allowed
variations in mineral compositions.
Only a few
minerals have a fixed composition – even quartz, which comes about as close as
possible to being purely SiO2, has trace amounts of other elements, yielding
varieties of quartz like
amethyst Fe3+
rose
quartz Ti4+
smoky
quartz structural defects
This raises
an issue of terminology:
major
elements are
fundamental to the mineral, control its structure and gross physical properties
minor
elements are
present in small amounts (up to a few %), usually as substitutes for major
elements
trace
elements are
present in extremely small amounts but are often responsible for mineral color.
We also
need to introduce the idea of mineral formulas, which is how we describe mineral
compositions. Conventions:
Cations
are listed before anions and anion complexes
Subscripts
outside of parentheses refer to everything inside
cations in same structural site are
grouped together
elements
separated by a comma inside parentheses are substitution pairs
Charges
must balance
Largest
cations are listed first (listed in order of decreasing coordination number)
Loosely bonded interstitial compounds are on the right; when
water occupies an interstitial site it is often separated from the main formula
by a dot.
EX: olivine (Mg,
Fe)2SiO4
natrolite Na2Al2Si3O10.2H2O
montmorillonite (Na, Ca)(Al,Mg)2(Si4O10)(OH)2.nH2O
We can also
write a single formula in different ways to convey different information. Let’s look at an oxide mineral that is
a type of spinel.
Idealized
formula Fe2ZnO4
The cations are Fe3+ and Zn2+, and they coordinate with O2-, which is the only anion.
Based on
knowledge of cation size, we can anticipate that Zn will be in 4-fold
(tetrahedral) coordination and Fe in 6-fold (octahedral) coordination,
consistent with the order in which they are written.
Structural
formula VIFe2IVZnO4
Often, a
structural site may be interchangeably occupied by different cations as part of
a solid solution series. In
this case, the interchangeable cations are grouped within parentheses. The spinel formula shown above can be
modified to show that the octahedral sites can hold either Fe3+ or
Mn3+ ions and the tetrahedral sites can hold either Zn2+
or Fe2+ ions.
General
formula (Fe, Mn)2(Zn,Fe)O4
Note that in
this formula, the cations in parentheses are conventionally assumed to be
listed in order of decreasing abundance… that is, Fe is more likely than Mn to
occupy the octahedral site, while Zn is more likely than Fe to occupy the
tetrahedral site.
Using certain
analytical techniques, it is possible to determine the proportion or relative
abundance of each type of cation occupying a substitution site in a given
sample. This information yields
the sample’s specific mineral formula, which could look something like this:
Specific
formula (Fe1.4 Mn0.6)(Zn0.8
Fe0.2)O4
In this
example, Fe3+ ions proportionally across the structure occupy 1.4 of every two filled
octahedral sites, while Mn3+ ions occupy the remaining 0.6 of every
two filled octahedral sites. Similarly,
Zn2+ ions proportionally occupy 0.8 of every filled tetrahedral
site, while Fe2+ ions occupy 0.2 of every filled tetrahedral site.
Solid
Solutions
The discussion above leads directly to a discussionof
substitutions of one element for another within the stable mineral structure
called isostructural substitutions. This process
is known as solid solution, defined in a mineral structure as specific atomic sites
that are occupied in variable proportions by two or more different chemical
elements.
Three
main factors determine whether or not solid solution is possible:
1.
Comparative size of ions (atoms, molecules) that are substituting for one
another This results directly from Pauling’s
first rule of radius ratios, in that ions that substitute must be able to
occupy the same interstitial site.
Generally, for this to happen the radius ratios must be within 15%;
substitution is unlikely when the radii differ by > 30%.
2.
The valence state (charge) of the ions involved in the substitution. This stipulation relates to
Pauling’s second rule, which involves electrical neutrality. If the substituting elements have the
same charge (Fe2+ and Mg2+; Na+ and K+),
then neutrality will be maintained.
If the charges are different (Al3+ and Si4+; Na+
and Ca2+), then another ionic substitution must take place to
maintain neutrality – this is called a coupled substitution, for example Ca2+Al3+
for Na+Si4+
3.
The temperature at which the substitution takes place. Substitution of ions of different size is favored by
elevated temperatures, where the structure is expanded and there is greater
tolerance for size variation.
Types
of substitution
Simple cationic/anionic: Ions of similar size and charge
substitute for each other.
Examples:
|
K = Na |
KCl – NaCl
(sylvite - halite); KAlSi3O8-NaAlSi3O8 (orthoclase – albite) |
|
Mg = Fe
(= Mn) |
Mg2SiO4
– Fe2SiO4 – Mn2SiO4 (forsterite –
fayalite - tephroite; olivine) MgSiO3
– FeSiO3 (enstatite – ferrosilite; pyroxene) |
|
Cl - Br |
KCl - KBr |
|
Fe = Zn |
(Zn, Fe)S (sphalerite) |
Depending
on the relative sizes of the ions involved, the solid solution may be either
partial (K = Na; ionic radii 1.46:1.08 in 6-fold coordination) or complete (Mg
= Fe; ionic radii 0.77:0.80 in 6-fold coordination).
Coupled
substitution: For
electrical neutrality to be maintained, substitution of two elements requires
an additional substitution.
Examples:
|
Fe2+
+ Ti4+ = 2Al3+ |
(Al, Ti)2O3 (corundum, var. sapphire) |
|
Ca2+Al3+
= Na+Si4+ |
CaAl2Si2O8-NaAlSi3O8 (plagioclase) |
|
Mg2+
+ 2Al3+ = 2Fe2+ + Ti4+ |
(Mg,
Fe)(Al, Ti)2O4 (spinel group) |
Interstitial
substitution: Between
some ions or ionic groups there may exist
structural voids.
Particularly where these have the form of channels (as in beryl and some
zeolites), they may be partially filled.
Example:
BERYL Be3Al2Si6O18
may contain substantial amounts of Li, Na, K, Rb through coupled substitutions
involving Si4+ and Al3+
Vacancy
solid solution: remember
that close packing of anions often creates more cation sites than can be
filled. Partial filling of these
sites forms another type of substitution.
A common example is the mineral amphibole, which has the end member
TREMOLITE []
Ca3Mg5Si8O22(OH)2
where []
represents a vacant site that may be filled using the coupled substitution
[]
+ Si4+ = Na+Al3+
Omission
solid solution: this
is the opposite of filling a vacancy, that is, creating one. An example is the substitution of the
large Pb2+ cation for the equally large K+ cation as
K+
+ K+ = Pb2+ + []
The result
of these substitutions is a wide variety of mineral and mineral formulas!!!
Crystallization
and polymorphs
So – how,
and why, do crystals form?
Crystals
typically form from a supersaturated solution, as we experimented with in lab. That solution may be an aqueous phase,
a magma, or a gas. During
metamorphism we also see examples of solid state crystallization (that is, one
crystal growing from another solid).
We may
create a supersaturated solution by changing the temperature, changing the
pressure, or changing the composition (by either adding or subtracting
components). We usually show
mineral stability fields using phase diagrams, as shown below for the system SiO2.
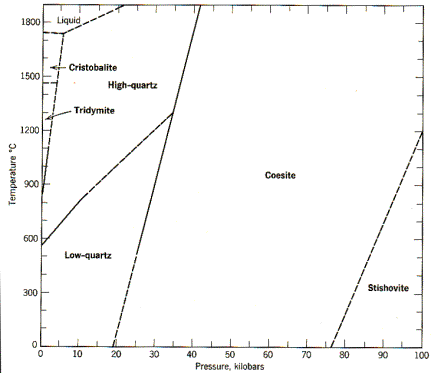
The figure shows graphically the ranges of pressure-temperature
conditions under which each of the different polymorphs are stable. At low
temperatures and pressures like those at the Earth's surface, low quartz is the
thermodynamically stable polymorph, accounting for its great abundance. As P and/or T change however, the low
quartz structure becomes unstable, and the SiO4
tetrahedra rearrange themselves to form new structures each of which has
different symmetry:
Polymorph Symmetry
low
quartz trigonal
high
quartz hexagonal
tridymite orthorhombic
cristobalite tetragonal
coesite monoclinic
stishovite tetragonal
You will
note from the figure that the reaction boundaries separating adjacent stability
fields are either quite P-dependent (roughly parallel to the T-axis, e.g., coesite
= stishovite), quite T-dependent (roughly parallel to the P-axis, e.g., high
quartz = liquid), or dependent on both P and T. As a rule, if a reaction is very pressure dependent, this
means that the polymorph on the high-P side of the boundary has a lower
molar volume that
that on the low-P side of the boundary.
This is illustrated by the transition from coesite (density = 2.93 g/cc)
to stishovite (density = 4.30 g/cc), which is accompanied by a nearly 50%
increase in density or a nearly 50% decrease in molar volume.
The phase transitions between the various polymorphs are of
two types with that between low quartz and high quartz being of a type referred
to as displacive
and all others being of a type known as reconstructive. The reconstructive transitions are most familiar and consist
of actual breaking of bonds and formation of new bonds in a different
configuration. Transitions of this type require considerable energy (enthalpy
of reaction) to break the bonds and, as a consequence, these reactions are
often sluggish leading to the common preservation of high-T or high-P forms at
conditions way outside of their stability fields. By contrast, the displacive
transitions do not require any breakage of bonds but instead, bonds and
polyhedra rotate to new positions with different symmetry. Transitions of this
type are extremely rapid and consequently, only the low-T, low-P forms are
preserved at the Earth's surface for us to collect.