Lecture 2 - Atoms and Elements
 1862-1870 Periodic table developed first by a geologist and
mineralogist Alexandre Beguyer de
Chancourtois (1862), who published a list of all of the known elements in order
of their atomic weights. He also
recognized that some elements had the same physical properties, thus
introducing the idea of periodicity.
In 1868 Dmitri Mendeleev expanded on the idea that there was a periodic
repetition of elements with similar physical and chemical properties. Ex. atoms numbered 3,11,19,37,55 (Li, Na, K, Rb, Cs) all soft, silvery white metals. All these elements are reactive, and
form perfect cubes when combined with chlorine. The resulting compounds (LiCl, NaCl, KCl, RbCl and CsCl) are
all colorless and display perfect cubic cleavage. In fact, the symmetry of his table was so compelling that he
recognized absence of elements where not yet discovered ...
1862-1870 Periodic table developed first by a geologist and
mineralogist Alexandre Beguyer de
Chancourtois (1862), who published a list of all of the known elements in order
of their atomic weights. He also
recognized that some elements had the same physical properties, thus
introducing the idea of periodicity.
In 1868 Dmitri Mendeleev expanded on the idea that there was a periodic
repetition of elements with similar physical and chemical properties. Ex. atoms numbered 3,11,19,37,55 (Li, Na, K, Rb, Cs) all soft, silvery white metals. All these elements are reactive, and
form perfect cubes when combined with chlorine. The resulting compounds (LiCl, NaCl, KCl, RbCl and CsCl) are
all colorless and display perfect cubic cleavage. In fact, the symmetry of his table was so compelling that he
recognized absence of elements where not yet discovered ...
1897 J.J. Thompson discovered electrons; soon after Rutherford recognized that atoms had a small
nucleus composed of protons and neutrons (to explain charge and mass).
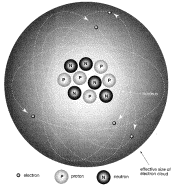
Bohr model of the atom: Bohr introduced the
concept of energy levels, known as quantum numbers. He believed that these levels were arranged as spherical shells, a geometry now known to be too restrictive
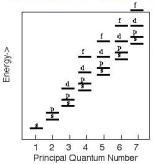
SUMMARY OF QUANTUM NUMBERS
1. The principle quantum number (n) determines the major energy
level. n = 1-7, with each level also designated by a le to Q, with K (n=1) being the lowest energy level and Q (n=7) the highest.: K is equivalent to (n=1). When an electron moves from a higher energy level to a lower one, energy is released as electromagnetic radiation (usually x-rays); this forms the basis for many of our analytical techniques. The
maximum number of electrons allowed at any one level is 2n2.
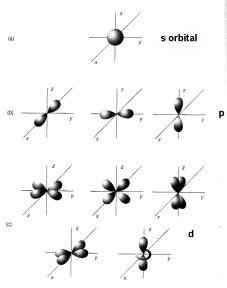 2. The angular momentum quantum
number (l) represents 'subshells' or orbitals, within each energy level. l varies from 0 to n-1, with higher values of l corresponding to higher
angular momentum. The
corresponding orbitals are s, p, d, and f for l = 0-3; these
may hold up to 2,6,10 and 14 electrons, respectively. Electrons fill orbitals from lowest to highest energy. The outermost electrons are called the alence electrons
2. The angular momentum quantum
number (l) represents 'subshells' or orbitals, within each energy level. l varies from 0 to n-1, with higher values of l corresponding to higher
angular momentum. The
corresponding orbitals are s, p, d, and f for l = 0-3; these
may hold up to 2,6,10 and 14 electrons, respectively. Electrons fill orbitals from lowest to highest energy. The outermost electrons are called the alence electrons
3. The magnetic quantum number (m) relates to the magnetic field generated by an electron with angular momentum and may be –1, 1
4. The spin quantum number relates to the intrinsic magnetism of the electron itself, and may be either -1/2 or +1/2
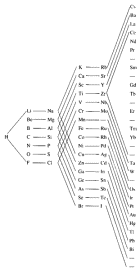
Modern
periodic table
- Periods are rows; the
number of the period indicates the orbitals occupied by electrons. EX: elements in 1st
period contain electrons in 1s orbital; elements in 2nd period have
electrons in 2s and 2p orbitals; elements in 3rd period have electrons in 3s and 3p … 4th to 7th periods have 10 extra
elements with electrons in d-orbitals.
6th and 7th periods have additional 14
elements with electrons in f-orbitals [listed separately at the bottom of the table
… includes rare earth elements and actinide series]
- Groups
-orbitals that are readily given up to produce cations. Elements near the right hand side of the table have nearly full orbitals and thus easily acquire additional electrons; these elements become nions.
Properties of elements in the middle of the table (the transition
elements) are
less predictable. Important
groups are the alkalis (I), the alkali earths (II), the halogens (VII),
and the transition elements (partially filled d- and f-orbitals, and
therefore may have unpredictable behavior).

IONS
 Atoms are most stable if electrons
completely fill energy levels and sublevels. For this reason, many atoms will either give up or accept
extra electrons in order to stabilize their configuration, thereby creating ions. The number of electrons gained or lost is referred to as the
valence, or valence
state. The process
of losing an
electron is called oxidation, while gaining an electron is reduction. When one or more electrons are
lost from the electron configuration of the atom, a cation is formed. When electrons are
added, an anion
is formed.
Atoms are most stable if electrons
completely fill energy levels and sublevels. For this reason, many atoms will either give up or accept
extra electrons in order to stabilize their configuration, thereby creating ions. The number of electrons gained or lost is referred to as the
valence, or valence
state. The process
of losing an
electron is called oxidation, while gaining an electron is reduction. When one or more electrons are
lost from the electron configuration of the atom, a cation is formed. When electrons are
added, an anion
is formed.
Energy required to removed the most weakly held electron is
known as the first ionization potential.
Note that the noble (inert) elements have the highest ionization energy,
while the alkalis (row 1 of the periodic table) have the lowest. However, a more common measure is that
of the ability of an atom in a crystal structure or molecule to attract
electrons to its outer shell - calculated from known bonds strengths, and known
as electronegativity (concept developed by Pauling).
Atoms with different electronegativity form ionic bonds with one another, and elements of
periodic table can be divided into two groups, electron donors (the metals, in the left-hand side of the
periodic table) and electron acceptors (the nonmetals, on the righthand side of the
periodic table). Elements with similar electronegativities form covalent
bonds (C).
Additionally, several elements are found in more than one valence state. EX: Fe can occur in a divalent
state Fe2+ (ferrous iron) or Fe3+
(ferric iron).
Sizes of ions
Anions are formed when atoms gain 1 or
more electrons
Cations are formed when atoms lose 1 or
more electrons
Anions
are thus generally larger than cations, and crystal structures can be envisaged
as large spheres packed around small spheres in such a way that the space
between spheres is minimized, and positive and negative charges are
balanced. Thus the chemical
classification system that we use is based primarily on the anion or anion
group.
Atomic
number and mass
Atomic number is the number of protons in an element's nucleus (Z) and, in a neutral atom, is also equal to the number of electrons. It is also close to the number of electrons in most ions. Most
important in controlling elemental properties.
Atomic
mass (A) is the
number of protons (Z) + number of neutrons (N). Most
elements have several different isotopes (same Z different N). Although chemists don't worry much about isotopes, very important in geology as climate tracers and for radioactive dating of geologic materials.
A
mole of an
element is defined as the amount of that element that has its weight in grams
equal to its atomic weight. Given by Avogadro's number (N): one mole of an element or compound always has 6.022 x 1023 atoms.
EX: Box 1.2 What is a mole of quartz?
SiO2
= 28.0855 + 2(15.994) = 60.0843 grams
Bonding in minerals
A
chemical bond exists when the forces acting between two elements are sufficient
to form a new aggregate or molecule.
There are four primary types of chemical bonds:
Covalent bonds involve shared electrons in outer shell; this means that an electron pair occupies two different orbitals simultaneously; such bonds are directional because of this orbital control. Covalent bonds are strong (diamond) and molecules with these
bonds tend to be electrical insulators.
Ionic
Metallic bonds are al'>acteristic of native metals, which are elements that easily lose their outer electrons. In a metal there are more bond sites (empty orbitals) than there are electron pairs to fill them. Detached electrons mover freely through the structure, thus metals are good electrical
conductors.
Van
der Waals; not important in most minerals (except graphite).
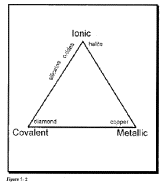 Type of bonding in minerals determines both the symmetry and the physical properties of minerals; the most important bond in many
minerals that we will examine is the Si-O bond, which is partly ionic and
partly covalent (called polar covalent); directionality of covalent part of bond is preserved.
Type of bonding in minerals determines both the symmetry and the physical properties of minerals; the most important bond in many
minerals that we will examine is the Si-O bond, which is partly ionic and
partly covalent (called polar covalent); directionality of covalent part of bond is preserved.

Effects
of size
As important as valence state is the relative size of the two ions (ionic
radius), which
dictates their separation. Note
that the attractive force increases as the atoms approach each other, with a
maximum when they are just touching.
As soon as their electron clouds overlap, repulsive forces become strong
(counteracts attractive energy).
The minimum energy configuration occurs where the distance between
centers of ions is equal to the sum of their ionic radii (closest packing).