Lecture 15 More metamorphic Rocks
REVIEW from
last time:
We’ve
defined metamorphic grade by sequences of index minerals in either pelitic
(muddy) rocks or basalts. For
example:
Low grade (slate) |
Medium grade (schist) |
High grade (gneiss) |
|
Chlorite Ź Biotite Ź |
Garnet Ź staurolite Ź kyanite [cordierite
Ź andalusite] |
Sillimanite |
This is
called a Barrovian
sequence (with the lower pressure Buchan sequence shown in brackets].
|
Amphibole type |
Stability Range |
Metamorphic Rock Name |
|
green
amphibole |
low T,
low P |
greenschist |
|
black
amphibole |
moderate
T,P |
amphibolite |
|
blue
amphibole |
low T,
high P |
blueschist |
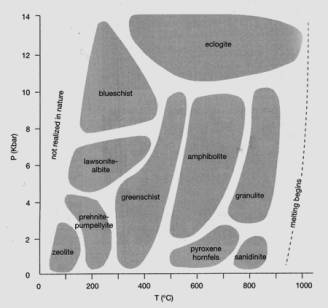
We use the
latter to define the primary metamorphic facies
Metamorphic
Reactions
1.
Solid-solid reactions
A simple
example of both the kinds of chemical reaction and their role in defining
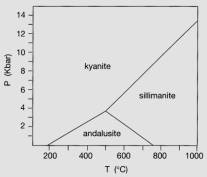
conditions of metamorphism is provided by the one component system Al2SiO5. This system consists of three minerals
– kyanite, andalusite, and sillimanite – each of which has the same chemical composition
but different structure. That is,
just like graphite and diamond, these minerals are polymorphs. The phase diagram for this system shows that each mineral is stable over a particular pressure
and temperature range. For
example, andalusite forms only at pressures less than those of the “triple
point”, that is, the single point in P-T space where all three phases can
coexist. Similarly, sillimanite
can form only at temperatures greater than that of the triple point. Additionally, this diagram shows that
progressive heating at low pressure (P < 4000 bars) produces first kyanite, then andalusite
then sillimanite. The transition
from kyanite to andalusite is known as the andalusite isograd, while the transition from
andalusite to sillimanite is called the sillimanite isograd.
Use of this
diagram requires that we have an equilibrium assemblage. One test of that is known as Gibbs
Phase Rule:
F
= C – P + 2
where F is
the “degrees of freedom”, C is the number of components (here, Al2SiO5),
and P is the number of phases; the “2” accounts for P, T. The phase rule says that, in the Al2SiO5
system, rocks containing one phase are divariant (2 degrees of freedom), rocks
with 2 phases are univariant (1 degree of freedom), and rocks containing all
three phases are invariant (they can equilibrate at only one P-T condition).
2.
Dehydration reactions
Sedimentary rocks, especially those
rich in clay minerals, may contain up to 6 wt% H2O. As these rocks undergo metamorphism,
resultant minerals contain increasingly less water. Many of the reactions that produce these minerals are dehydration
reactions, that is,
reactions that break down hydrous minerals to produce minerals with lower (or
no) water, thus releasing water in the process. All of the reactions that produce index minerals during the
progressive metamorphism of shales (e.g., biotite, garnet, staurolite, kyanite,
sillimanite) are dehydration reactions.
EX: KAl2Si3AlO10(OH)2
+ SiO2 = KAlSi3O8 + Al2SiO5
+ H2O
muscovite
+
quartz = K-feldspar + aluminosilicate
+ water
You can
also have decarbonation reactions, such as CaCO3 + SiO2 =
CaSiO3 + CO2
NOTE:
The Transition from Metamorphism to Magmatism
An important dehydration reaction is one that occurs only at
very high grades of metamorphism (at ~ 700ŻC). It involves the breakdown of white mica (muscovite) in the
presence of quartz to produce K-feldspar, sillimanite, and H2O. Remember (from the igneous section of
the class) that “normal” continental geothermal gradients are not sufficiently
high to produce melt. However,
with H2O present, melting may occur at much lower temperatures. If we look at a diagram for dry and wet
melting of muscovite + water, we
see that the “wet” melting curve permits melting at ~ 700ŻC and 5000 bars
pressure. This melting mechanism
is probably responsible for formation of granite deep in the continental crust,
probably the origin of many large batholiths (such as the Idaho Batholith).
3.
Continuous reactions
Another
important type of metamorphic reaction is one that simply exchanges cations
among solid solution minerals that coexist in a metamorphic rock. For example, Mg2+ and Fe2+
can be exchanged between garnet and biotite:
KMg3AlSi3O10(OH)2
+ Fe3Al2Si3O12 ćŹ KFe3AlSi3O10(OH)2
+ Mg3Al2Si3O12
Mg-biotite
Fe-garnet Fe-biotite Mg-garnet
This
reaction is virtually independent of pressure, but strongly dependent on
temperature. Thus, the composition
(Fe/Mg ratio) of coexisting garnet and biotite in a metamorphic rock is
primarily determined by temperature – for this reason, this reaction can be
used as a geothermometer, if properly calibrated in the lab.
Similarly,
reactions that are strongly dependent on pressure can be used as geobarometers. By careful use of geothermometers and geobarometers, metamorphic
petrologists are often able to constrain T to within about 30ŻC and P to within
500 bars.