Lecture 1
Earth Materials - Minerals and
Rocks
Minerals: basic
compounds of the solid earth. ~
3000 known; these 3000 contain all of the chemical elements of which the earth
is made.
Rocks: physical
mixtures of minerals. Most of the
rocks that make up the earth are composed of only ~ 50 of the known minerals
Why
study? Minerals occur in nearly all inorganic
materials of everyday life, and are thus important economically, aesthetically
and scientific terms.
ECONOMIC Many minerals
have economic value - early on were used for pigments, gemstones for
ornamentation, clay for bricks, iron for utensils. Not until the 19th century, however, were metals mined and
extracted.
ore minerals - minerals from which valuable
metallic elements can be extracted
industrial minerals - nonmetallic materials used
manufacturing (EX: insulators, ceramics, glass, cement, fertilizers, etc.)
gangue minerals - those minerals not considered
useful but which must be mined anyway
Some minerals are now considered
"strategic" - [ex. Al and Cr in WWII]
Synthetic minerals are now important
in materials science (Ex. high-T superconductors)
AESTHETIC Gemstones have been
important throughout the history of most cultures, and precious metals, prized
for their aesthetic values, form the basis of most economies.
SCIENTIFIC Minerals are the building blocks
of all solid parts of the universe, and are therefore fundamental to all
aspects of the geological sciences.
Some examples:
- petrologists
use individual
minerals that form under restricted conditions of pressure and temperature to
map physical conditions within the Earth
- geophysicists studying the Earth's interior need
to understand how minerals behave under pressure, and how they transmit seismic
waves
- geochemists
use changes in
water chemistry to understand processes of weathering and contamination by
interaction with mineral surfaces
Minerals are also important to a
number of related scientific disciplines, including
- agricultural
sciences (soils)
- hydrology (important for understanding
development of aquifers and aquitards)
- materials
science
(development of synthetic minerals with specific properties)
- medical
science (e.g.,
asbestos)
- biological
sciences (may have
been important as templates for the origin of life)
Definition of a mineral:
A mineral is a naturally
occurring, inorganic, homogeneous solid with a definite chemical composition
and an ordered atomic arrangement
- naturally occuring - this modifier in the definition is considered superfluous by some, but
is an important distinction to others (especially gemologists)
- homogeneous solid a single, solid substance (phase)
that cannot be physically separated into distinct compounds; this distinguishes
minerals from rocks that can be disaggregated into individual mineral
constituents
- definite chemical composition -al'>ineral is a chemical element or compound whose
composition can be represented by a chemical formula (e.g., SiO2). The compositions of minerals are either
fixed, or vary
within specified limits (EX: the mineral olivine may vary in composition from
Mg2SiO4 to Fe2SiO4). The composition dictates the mineral
structure, which in turn controls the physical properties of the mineral
- ordered atomic arrangement - nearly all minerals are crystalline. It means that the internal structure
is characterized by periodic or predictable arrays of atoms, ions, or molecules
... this ordered arrangement of atoms is often expressed in the symmetry of the
external form. It also means that
different minerals may have the same composition but different structures (EX:
graphite and diamond).
The crystalline state of matter
is the most fundamental property of minerals
In fact, the word 'crystal' is an anglicized version of the
Greek word for ice, and was generally employed through the Middle Ages for
describing quartz - 'rock crystal' - as quartz was considered to be permanently
solidified water. The word has
persisted for any quartz clear enough for ornaments (e.g., New Age uses) or
glass.
Aspects of Earth Materials that
we will cover this term:
- crystal chemistry - the chemical nature of minerals
- crystallography - the study of symmetry and the
internal order of crystals
- mineral physics -
the physical properties of minerals
- petrology -al'>
study of rocks and the minerals that form them
A bit of history
Very
early man recognized natural pigments and used them in cave paintings. Stone Age man knew something about the
hardness of minerals, and about mineral cleavage (how they break) - jadeite
commonly used as tools. The mining
and smelting of minerals for iron, copper, bronze, lead, silver probably dates
back to > 4000 yrs. However,
there is no written record of any of this.
300 BC Theophrastus (pupil of Aristotle)
wrote On Stones
23-79 AD Pliny the Elder recorded a great deal of natural
history, and described a number of minerals mined as gemstones, ores, and
pigments. His are the earliest
comments on crystal form and the quality of crystal faces. Books 33-37 of his volume on Natural
History cover such topics as precious metals (not only mining and uses but also
manŐs greed and exploitation of mineral resources); use of minerals for pigments
and painting; mining
and metallurgy; marble and other materials used in
sculpture; and precious stones.
 1556 AD Georgius
Agricola (Georg
Bauer; German physician) published De Re Metallica, in which "I have omitted all those things which I have not myself seen, or have not read or heard of from persons upon whom I can rely." He is
considered the founder of geology as a discipline, and his work contributed to
the fields of mining geology, metallurgy, mineralogy, structural geology and
paleontology. This book became a
handbook of mining practices for the next two centuries, and laid the framework
of stratigraphic principles. In
other books he developed a classification systems for minerals based on their
physical properties, gave them standardized names and recorded where they could
be found.
1556 AD Georgius
Agricola (Georg
Bauer; German physician) published De Re Metallica, in which "I have omitted all those things which I have not myself seen, or have not read or heard of from persons upon whom I can rely." He is
considered the founder of geology as a discipline, and his work contributed to
the fields of mining geology, metallurgy, mineralogy, structural geology and
paleontology. This book became a
handbook of mining practices for the next two centuries, and laid the framework
of stratigraphic principles. In
other books he developed a classification systems for minerals based on their
physical properties, gave them standardized names and recorded where they could
be found.
1611 AD Kepler speculated on the planar
arrangement of spheres as an explanation for the symmetry of snowflakes.
1669 Nicolaus
Steno recognized
that the interfacial angles of quartz crystals were the same regardless of the
size or shape of the crystal. 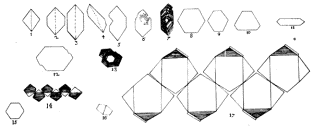 This led to his hypothesis of the
constancy of interfacial angles, with the basis that there is an underlying pattern of symmetry to
crystal forms. He concluded that
processes of crystal growth may allow different faces to dominate under
different growth conditions - this results in crystals of the same composition having
very different external forms.
SIGNIFICANCE OF CRYSTAL FORM
This led to his hypothesis of the
constancy of interfacial angles, with the basis that there is an underlying pattern of symmetry to
crystal forms. He concluded that
processes of crystal growth may allow different faces to dominate under
different growth conditions - this results in crystals of the same composition having
very different external forms.
SIGNIFICANCE OF CRYSTAL FORM
1768 AD Carolus
Linnaeus developed
a mineral classification based primarily on external form. Throughout 18th
century, studies of chemistry and mineralogy closely linked, as chemists worked
with minerals as their raw materials.
Saw the discovery of many new minerals and, with that, identification of
many new elements (cobalt, nickel, manganese, tungsten, molybdenum, uranium
...)
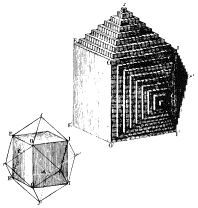 1784 AD The French mineralogist Rene Hauy recognized that the perfection of
external form, the symmetry possessed by crystals, and the existence of perfect
cleavage must be manifestations of the internal structure of minerals
(involving planes of atoms). In
his study of calcite crystals Hauy defined axes of reference for a few
crystals, and recognized that all faces on a crystal cut those axes at simple
multiples - this led to his conception of crystal structures made up of
identical "integral molecules" ... turns out that this concept is
similar to the modern concept of a space lattice. This work provided the relationship between
crystallography and mineralogy.
1784 AD The French mineralogist Rene Hauy recognized that the perfection of
external form, the symmetry possessed by crystals, and the existence of perfect
cleavage must be manifestations of the internal structure of minerals
(involving planes of atoms). In
his study of calcite crystals Hauy defined axes of reference for a few
crystals, and recognized that all faces on a crystal cut those axes at simple
multiples - this led to his conception of crystal structures made up of
identical "integral molecules" ... turns out that this concept is
similar to the modern concept of a space lattice. This work provided the relationship between
crystallography and mineralogy.
1830 AD Johan Hessel proved that geometric constraints
limit the number of crystal classes to 32, and that only 2-, 3-, 4-, and 6-fold
axes of rotational symmetry are possible in minerals.
1837 AD James D Dana (Yale) completed the 1st edition of
A System of Mineralogy, a book which, in the 4th edition, introduced the chemically based
classification system that we still use.
1848 Auguste Bravais proposed the existence of 32 crystal
classes He also
proposed that there were only 14 space lattices (that is, he demonstrated that
only 14 regular patterns in space can result form the periodic arrangement of
points) - his work was the forerunner of space group theory. He also perceived that the 14 space
lattices consisted of 7 different lattice symmetries, which correspond to 7
crystal systems.
 1870 AD Petrographic (polarizing) microscope
invented in mid-1800s ... brought to this country by G. Huntington Williams
(Johns Hopkins). This allowed
determination of the optical properties of minerals, and also made possible the
study of fine-grained rocks...
1870 AD Petrographic (polarizing) microscope
invented in mid-1800s ... brought to this country by G. Huntington Williams
(Johns Hopkins). This allowed
determination of the optical properties of minerals, and also made possible the
study of fine-grained rocks...
1895 AD Wilhelm
Roentgen discovered
xrays; he showed that xrays were able to pass through materials that are opaque
to light, invisible to the human eye, and could be recorded on photographic
paper. For this work Roentgen was
awarded the first Nobel Prize in physics in 1901.
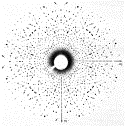 1912 AD The physicist Max von Laue first demonstrated that xrays were
diffracted by a crystal structure (in his case, copper sulphate). The implications of this observation
were that the wavelengths of xrays were comparable with the spacing between
atoms in crystals. This was the
start of an entire field devoted to the determination of crystal structure.
1912 AD The physicist Max von Laue first demonstrated that xrays were
diffracted by a crystal structure (in his case, copper sulphate). The implications of this observation
were that the wavelengths of xrays were comparable with the spacing between
atoms in crystals. This was the
start of an entire field devoted to the determination of crystal structure.
1913 AD The
father-son team W. Lawrence and W. Henry Bragg used diffraction to analyze the
first crystal structure, that is, the precise position of atoms in the
structure was ascertained, along with the distances and angles between
atoms. This work allowed
inferences about atomic size and bond strengths, and thus won the Braggs the
Nobel Prize in 1915.
 The twentieth century has seen a
dramatic increase in the 'tools' available for studying minerals - XRD, XRF,
electron microprobe, electron microscope, spectroscopic techniques, and, most
recently, computer-assisted tomography, TEM and atomic force microscopy, where
individual atoms can be imaged...
The twentieth century has seen a
dramatic increase in the 'tools' available for studying minerals - XRD, XRF,
electron microprobe, electron microscope, spectroscopic techniques, and, most
recently, computer-assisted tomography, TEM and atomic force microscopy, where
individual atoms can be imaged...
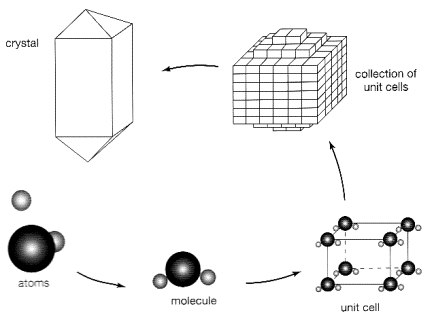
We now realize that the internal
structure of any mineral can be described as a 3-D array of positive (cations)
and negative (anions) ions built up by a regular repetition of the unit cell. Symmetry and form of a crystal are determined by the shape
of that unit cell. The chemical
formula of a mineral expresses the relative
proportions of its constitutive elements.